Abstract
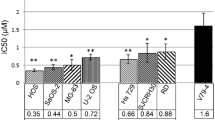
Ruthenium(II)/benzonitrile complexes have demonstrated promising anticancer properties. Considering that there are no specific therapies for treating sarcoma, we decided to evaluate the cytotoxic, genotoxic, and lethal effects of cis-[RuCl(BzCN)(phen)(dppb)]PF6 (BzCN = benzonitrile; phen = 1,10-phenanthroline; dppb = 1,4-bis-(diphenylphosphino)butane), as well as the mechanism of cell death induction that occurs against murine sarcoma-180 tumor. Thus, MTT assay was applied to assess the ruthenium cytotoxicity, showing that the compound is a more potent inhibitor for the sarcoma-180 tumor cell viability than normal cells (lymphocytes). The comet assay indicated low genotoxic for normal cells. cis-[RuCl(BzCN)(phen)(dppb)]PF6 also showed moderate lethality in Artemia salina. The complex induced cell cycle arrest in the G0/G1 phase in sarcoma-180 cells. In addition, the complex caused S180 cells to die by apoptosis by an increase in Annexin-V-positive cells and morphological changes typical of apoptotic cells. Additionally, cis-[RuCl(BzCN)(phen)(dppb)]PF6 increased the gene expression of Bax, Casp3, and Tp53 in S180 cells. By using a western blot, we observed an increased protein level of TNF-R2, Bax, and p21. In conclusion, cis-[RuCl(BzCN)(phen)(dppb)]PF6 is active and selective for sarcoma-180 cells, leading to cell cycle arrest at the G0/G1 and cell death through a caspases-mediated and Tp53/p21-mediated pathway.







Similar content being viewed by others
References
Demetri GD, Antonia S, Benjamin RS, Bui MM, Casper ES, Conrad EU, DeLaney TF, Ganjoo KN, Heslin MJ, Hutchinson RJ, Kane JM (2010) Soft tissue sarcoma. J Natl Compr Cancer Netw 8:630–674. https://doi.org/10.6004/jnccn.2010.0049
Fruehauf S, Veldwijk MR, Berlinghoff S, Basara N, Baum C, Flasshove M, Hegewisch-Becker S, Kröger N, Licht T, Moritz T, Hengge UR (2002) Gene therapy for sarcoma. Cells Tissues Organs 172:133–144. https://doi.org/10.1159/000065614
Milano A, Apice G, Ferrari E, Fazioli F, de Rosa V, de Luna AS, Iaffaioli RV, Caponigro F (2006) New emerging drugs in soft tissue sarcoma. Crit Rev Oncol Hematol 59:74–84. https://doi.org/10.1016/j.critrevonc.2005.12.002
Süss-Fink G (2010) Arene ruthenium complexes as anticancer agents. Dalton Trans 39:1673–1688. https://doi.org/10.1039/B916860P
Sabale PM, Patel J, Patel Y, Patel R (2012) Metal complexes: current trends and future potential. Int J Pharm Chem Biol Sci 2:251–265
Silveira-Lacerda EP, Vilanova-Costa CA, Hamaguchi A, Pavanin LA, Goulart LR, Homsi-Brandenburgo MI, dos Santos WB, Soares AM, Nomizo A (2010) The ruthenium complex cis-(dichloro) tetraammineruthenium (III) chloride presents selective cytotoxicity against murine B cell lymphoma (A-20), murine ascitic sarcoma 180 (S-180), human breast adenocarcinoma (SK-BR-3), and human T cell leukemia (Jurkat) tumor cell lines. Biol Trace Elem Res 135:98–111. https://doi.org/10.1007/s12011-009-8498-3
Thota S, Rodrigues DA, Crans DC, Barreiro EJ (2018) Ru (II) compounds: next-generation anticancer metallotherapeutics? J Med Chem 61:5805–5821. https://doi.org/10.1021/acs.jmedchem.7b01689
Zeng L, Gupta P, Chen Y, Wang E, Ji L, Chao H, Chen ZS (2017) The development of anticancer ruthenium (II) complexes: from single molecule compounds to nanomaterials. Chem Soc Rev 46:5771–5804. https://doi.org/10.1039/C7CS00195A
Pereira FC, Lima BA, de Lima AP, Pires WC, Monteiro T, Magalhaes LF, Costa W, Graminha AE, Batista AA, Ellena J, Siveira-Lacerda ED (2015) Cis-[RuCl (BzCN)(N–N)(P–P)] PF6 complexes: Synthesis and in vitro antitumor activity:(BzCN= benzonitrile; N–N= 2, 2′-bipyridine; 1, 10-phenanthroline; P–P= 1, 4-bis (diphenylphosphino) butane, 1, 2-bis (diphenylphosphino) ethane, or 1, 1′-(diphenylphosphino) ferrocene). J Inorg Biochem 149:91–101. https://doi.org/10.1016/j.jinorgbio.2015.03.011
Lima AP, Pereira FC, Almeida MA, Mello FM, Pires WC, Pinto TM, Delella FK, Felisbino SL, Moreno V, Batista AA, de Paula S-LE (2014) Cytoxicity and apoptotic mechanism of ruthenium (II) amino acid complexes in sarcoma-180 tumor cells. PLoS One 9:e105865. https://doi.org/10.1371/journal.pone.0105865
Magalhaes LF, Mello-Andrade F, Pires WC, Silva HD, da Silva PF, Macedo LM, de Castro CH, Carneiro CC, Cardoso CG, de Melo Reis PR, de Oliveira LC (2017) cis-[RuCl (BzCN)(bipy)(dppe)] PF6 induces anti-angiogenesis and apoptosis by a mechanism of caspase-dependent involving DNA damage, PARP activation, and Tp53 induction in Ehrlich tumor cells. Chem Biol Interact 278:101–113. https://doi.org/10.1016/j.cbi.2017.09.013
Gill MR, Harun SN, Halder S, Boghozian RA, Ramadan K, Ahmad H, Vallis KA (2016) A ruthenium polypyridyl intercalator stalls DNA replication forks, radiosensitizes human cancer cells and is enhanced by Chk1 inhibition. Sci Rep 6:31973. https://doi.org/10.1038/srep31973
Takarada JE, Guedes AP, Correa RS, Silveira-Lacerda ED, Castelli S, Iacovelli F, Deflon VM, Batista AA, Desideri A (2017) Ru/Fe bimetallic complexes: Synthesis, characterization, cytotoxicity and study of their interactions with DNA/HSA and human topoisomerase IB. Arch Biochem Biophys 636:28–41. https://doi.org/10.1016/j.abb.2017.10.015
Kapitza S, Jakupec MA, Uhl M, Keppler BK, Marian B (2005) The heterocyclic ruthenium (III) complex KP1019 (FFC14A) causes DNA damage and oxidative stress in colorectal tumor cells. Cancer Lett 226:115–121. https://doi.org/10.1016/j.canlet.2005.01.002
Popolin CP, Reis JP, Becceneri AB, Graminha AE, Almeida MA, Corrêa RS, Colina-Vegas LA, Ellena J, Batista AA, Cominetti MR (2017) Cytotoxicity and anti-tumor effects of new ruthenium complexes on triple negative breast cancer cells. PLoS One 12:e0183275. https://doi.org/10.1371/journal.pone.0183275
Mello-Andrade F, da Costa WL, Pires WC, Pereira FD, Cardoso CG, Lino-Junior RD, Irusta VR, Carneiro CC, de Melo-Reis PR, Castro CH, Almeida MA (2017) Antitumor effectiveness and mechanism of action of Ru (II)/amino acid/diphosphine complexes in the peritoneal carcinomatosis progression. Tumor Biol 39:1010428317695933. https://doi.org/10.1177/1010428317695933
Pires WC, Lima BA, de Castro PF, Lima AP, Mello-Andrade F, Silva HD, da Silva MM, Colina-Vegas L, Ellena J, Batista AA, Silveira-Lacerda EP (2018) Ru (II)/diphenylphosphine/pyridine-6-thiolate complexes induce S-180 cell apoptosis through intrinsic mitochondrial pathway involving inhibition of Bcl-2 and p53/Bax activation. Mol Cell Biochem 438:199–217. https://doi.org/10.1007/s11010-017-3129-3
Molina-Salinas GM, Said-Fernández S (2006) A modified microplate cytotoxicity assay with brine shrimp larvae (Artemia salina). Pharmacologyonline 3:633–638
Amarante CB, Müller AH, Póvoa MM, Dolabela MF (2011) Estudo fitoquímico biomonitorado pelos ensaios de toxicidade frente à Artemia salina e de atividade antiplasmódica do caule de aninga (Montrichardia linifera). Acta Amazon 41
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191. https://doi.org/10.1016/0014-4827(88)90265-0
Kobayashi HA (1995) Comparison between manual microscopic analysis and computerized image analysis in the single cell gel electrophoresis. MMS Commun 3:103–115
Bronikowska J, Szliszka E, Jaworska D, Czuba ZP, Krol W (2012) The coumarin psoralidin enhances anticancer effect of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). Molecules 17:6449–6464. https://doi.org/10.3390/molecules17066449
Rahman SA, Nur S, Abdul Wahab N, Malek A, Nurestri S (2013) In vitro morphological assessment of apoptosis induced by antiproliferative constituents from the rhizomes of Curcuma zedoaria. Evid Based Complement Alternat Med:2013. https://doi.org/10.1155/2013/257108
Rogalska A, Marczak A, Gajek A, Szwed M, Śliwińska A, Drzewoski J, Jóźwiak Z (2013) Induction of apoptosis in human ovarian cancer cells by new anticancer compounds, epothilone A and B. Toxicol in Vitro 27:239–249. https://doi.org/10.1016/j.tiv.2012.09.006
Xie YY, Li ZZ, Lin GJ, Huang HL, Wang XZ, Liang ZH, Jiang GB, Liu YJ (2013) DNA interaction, cytotoxicity, apoptotic activity, cell cycle arrest, reactive oxygen species and mitochondrial membrane potential assay induced by ruthenium (II) polypyridyl complexes. Inorg Chim Acta 405:228–234. https://doi.org/10.1016/j.ica.2013.06.002
Wang JQ, Zhang PY, Qian C, Hou XJ, Ji LN, Chao H (2014) Mitochondria are the primary target in the induction of apoptosis by chiral ruthenium (II) polypyridyl complexes in cancer cells. J Biol Inorg Chem 19:335–348. https://doi.org/10.1007/s00775-013-1069-2
Kokkali V, Katramados I, Newman JD (2011) Monitoring the effect of metal ions on the mobility of Artemia salina nauplii. Biosensors 1:36–45. https://doi.org/10.3390/bios1020036
Anchuri SS, Thota S, Yerra R, Devarakonda KP, Dhulipala S (2012) Novel mononuclear ruthenium(II) compounds in cancer therapy. Asian Pac J Cancer Prev 13(7):3293–3298. https://doi.org/10.7314/APJCP.2012.13.7.3293
Moshi MJ, Innocent E, Magadula JJ, Otieno DF, Weisheit A, Mbabazi PK, Nondo RS (2010) Brine shrimp toxicity of some plants used as traditional medicines in Kagera region, north western Tanzania. Tanzan J Health Res 12:63–67. https://doi.org/10.4314/thrb.v12i1.56287
Trzeciak A, Kowalik J, Malecka-Panas E, Drzewoski J, Wojewódzka M, Iwanenko T, Blasiak J (2000) Genotoxicity of chromium in human gastric mucosa cells and peripheral blood lymphocytes evaluated by the single cell gel electrophoresis (comet assay). Med Sci Monit 6:24–29
Sekihashi K, Yamamoto A, Matsumura Y, Ueno S, Watanabe-Akanuma M, Kassie F, Knasmüller S, Tsuda S, Sasaki YF (2002) Comparative investigation of multiple organs of mice and rats in the comet assay. Mutat Res Genet Toxicol Environ Mutagen 517:53–75. https://doi.org/10.1016/S1383-5718(02)00034-7
Ribeiro AD, da Silva CC, de Castro Pereira F, de Lima AP, Vilanova-Costa CA, Aguiar SS, Pavanin LA, da Cruz AD, de Paula Silveira-Lacerda E (2009) Mutagenic and genotoxic effects of cis-(dichloro) tetraammineruthenium (III) chloride on human peripheral blood lymphocytes. Biol Trace Elem Res 130:249–261. https://doi.org/10.1007/s12011-009-8334-9
Hata T, Ogawa T, Yokoyama T, Fukushige S, Horii A, Furukawa T (2004) DSCP1, a novel TP53-inducible gene, is upregulated by strong genotoxic stresses and its overexpression inhibits tumor cell growth in vitro. Int J Oncol 24(3):513–520. https://doi.org/10.3892/ijo.24.3.513
Li L, Wong YS, Chen T, Fan C, Zheng W (2012) Ruthenium complexes containing bis-benzimidazole derivatives as a new class of apoptosis inducers. Dalton Trans 41:1138–1141. https://doi.org/10.1039/C1DT11950H
Kasper C, Alborzinia H, Can S, Kitanovic I, Meyer A, Geldmacher Y, Oleszak M, Ott I, Wölfl S, Sheldrick WS (2012) Synthesis and cellular impact of diene–ruthenium (II) complexes: a new class of organoruthenium anticancer agents. J Inorg Biochem 106:126–133. https://doi.org/10.1016/j.jinorgbio.2011.08.027
Yang X, Chen L, Liu Y, Yang Y, Chen T, Zheng W, Liu J, He QY (2012) Ruthenium methylimidazole complexes induced apoptosis in lung cancer A549 cells through intrinsic mitochondrial pathway. Biochimie 94:345–353. https://doi.org/10.1016/j.biochi.2011.07.025
Chen Y, Qin MY, Wu JH, Wang L, Chao H, Ji LN, Xu AL (2013) Synthesis, characterization, and anticancer activity of ruthenium (II)-β-carboline complex. Eur J Med Chem 70:120–129. https://doi.org/10.1016/j.ejmech.2013.09.051
Costa CO, Neto JH, Baliza IR, Dias RB, Valverde LD, Vidal MT, Sales CB, Rocha CA, Moreira DR, Soares MB, Batista AA (2017) Novel piplartine-containing ruthenium complexes: synthesis, cell growth inhibition, apoptosis induction and ROS production on HCT116 cells. Oncotarget 8:104367. https://doi.org/10.18632/oncotarget.22248
Özgen Ü, Savaşan S, Buck S, Ravindranath Y (2000) Comparison of DiOC6 (3) uptake and annexin V labeling for quantification of apoptosis in leukemia cells and non-malignant T lymphocytes from children. Cytometry 42:74–78. https://doi.org/10.1002/(SICI)1097-0320(20000215)42:1<74::AID-CYTO11>3.0.CO;2-6
De Lima AP, Castro Pereira F, Vilanova-Costa CA, Ribeiro AD, Pavanin LA, Dos Santos WB, Silveira-Lacerda EP (2010) The ruthenium complex cis-(dichloro) tetrammineruthenium (III) chloride induces apoptosis and damages DNA in murine sarcoma 180 cells. J Biosci 35:371–378. https://doi.org/10.1007/s12038-010-0042-2
Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry WS, Fulda S, Gottlieb E (2012) Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ 19:107. https://doi.org/10.1038/cdd.2011.96
Kumar S (2007) Caspase function in programmed cell death. Cell Death Differ 14:32. https://doi.org/10.1038/sj.cdd.4402060
Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR (2010) The BCL-2 family reunion. Mol Cell 37:299–310. https://doi.org/10.1016/j.molcel.2010.01.025
Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D (1993) p21 is a universal inhibitor of cyclin kinases. Nature 366:701. https://doi.org/10.1038/366701a0
Brady HJ, Gil-Gómez G (1998) Molecules in focus Bax. The pro-apoptotic Bcl-2 family member, Bax. Int J Biochem Cell Biol 30:647–650. https://doi.org/10.1016/S1357-2725(98)00006-5
Gil-Gómez G, Berns A, Brady HJ (1998) A link between cell cycle and cell death: Bax and Bcl-2 modulate Cdk2 activation during thymocyte apoptosis. EMBO J 17:7209–7218. https://doi.org/10.1093/emboj/17.24.7209
Kook S, Gurevich VV, Gurevich EV (2014) Arrestins in apoptosis. In: Arrestins-pharmacology and therapeutic potential. Springer, Berlin, pp 309–339
Prives C, Hall PA (1999) The p53 pathway. J Pathol 187:112–126. https://doi.org/10.1002/(SICI)1096-9896(199901)187:1<112::AID-PATH250>3.0.CO;2-3
Gaiddon C, Jeannequin P, Bischoff P, Pfeffer M, Sirlin C, Loeffler JP (2005) Ruthenium (II)-derived organometallic compounds induce cytostatic and cytotoxic effects on mammalian cancer cell lines through p53-dependent and p53-independent mechanisms. J Pharmacol Exp Ther 315:1403–1411. https://doi.org/10.1124/jpet.105.089342
Pop C, Salvesen GS (2009) Human caspases: activation, specificity, and regulation. J Biol Chem 284:21777–21781. https://doi.org/10.1074/jbc.R800084200
Yuan J (1997) Transducing signals of life and death. Curr Opin Cell Biol 9:247–251. https://doi.org/10.1016/S0955-0674(97)80069-5
Lee P, Sata M, Lefer DJ, Factor SM, Walsh K, Kitsis RN (2003) Fas pathway is a critical mediator of cardiac myocyte death and MI during ischemia-reperfusion in vivo. Am J Phys Heart Circ Phys 284:456–463. https://doi.org/10.1152/ajpheart.00777.2002
Goretsky T, Dirisina R, Sinh P, Mittal N, Managlia E, Williams DB, Posca D, Ryu H, Katzman RB, Barrett TA (2012) p53 mediates TNF-induced epithelial cell apoptosis in IBD. Am J Pathol 181:1306–1315. https://doi.org/10.1016/j.ajpath.2012.06.016
Zhang F, Yang Y, Smith T, Kau SW, McConathy JM, Esteva FJ, Kuerer HM, Symmans WF, Buzdar AU, Hortobagyi GN, Pusztai L (2003) Correlation between HER-2 expression and response to neoadjuvant chemotherapy with 5-fluorouracil, doxorubicin, and cyclophosphamide in patients with breast carcinoma. Cancer 97:1758–1765. https://doi.org/10.1002/cncr.11245
Rokhlin OW, Gudkov AV, Kwek S, Glover RA, Gewies AS, Cohen MB (2000) p53 is involved in tumor necrosis factor-α-induced apoptosis in the human prostatic carcinoma cell line LNCaP. Oncogene 19:1959. https://doi.org/10.1038/sj.onc.1203453
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All subjects enrolled in this research filled in an Informed Consent form, which was approved by the Ethics Committee of the Universidade Federal do Goiás (UFG), and this protocol (n° 043/2007) was found to be acceptable by the committee.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 219 kb)
Rights and permissions
About this article
Cite this article
Faria, R.S., Silva, H.D., Mello-Andrade, F. et al. Ruthenium(II)/Benzonitrile Complex Induces Cytotoxic Effect in Sarcoma-180 Cells by Caspase-Mediated and Tp53/p21-Mediated Apoptosis, with Moderate Brine Shrimp Toxicity. Biol Trace Elem Res 198, 669–680 (2020). https://doi.org/10.1007/s12011-020-02098-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02098-8