Abstract
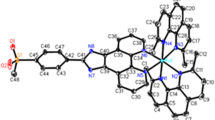
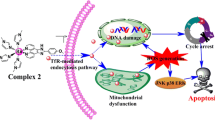
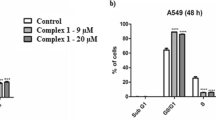
The aim of this work was the synthesis, characterization, and cytotoxicity evaluation of three new Ru(II) complexes with a general formula [Ru(Spy)(bipy)(P-P)]PF6 [Spy = pyridine-6-thiolate; bipy = 2,2′-bipyridine; P-P = 1,2-bis(diphenylphosphine)ethane (1); 1,3-bis(diphenylphosphine) propane (2); and 1,1′-bis(diphenylphosphino)ferrocene] (4). Complex (3) with the 1,4-bis(diphenylphosphine)butane ligand, already known from the literature, was also synthesized, to be better studied here. The cytotoxicities of the complexes toward two kinds of cancerous cells (K562 and S-180 cells) were evaluated and compared to normal cells (L-929 and PBMC) by MTT assay. The complex [Ru(Spy)(bipy)(dppb)]PF6 (3) was selected to study both the cellular and molecular mechanisms underlying its promising anticancer action in S-180 cells. The results obtained from this study indicated that complex (3) induces cell cycle arrest in the G0/G1 phase in S-180 cells associated with a decrease in the number of cells in S phase. After 24 and 48 h of exposure to complex (3), the cell viability decreased when compared to the negative control. Complex (3) does not appear to be involved in the DNA damage, but induced changes in the mitochondrial membrane potential in S-180 cells. Furthermore, there was also an increase in the gene expression of Bax, Caspase 9, and Tp53. According to our results, complex (3) induces cell apoptosis through p53/Bax-dependent intrinsic pathway and suppresses the expression of active antiapoptotic Bcl-2 protein.











Similar content being viewed by others
References
Demetri GD, Antonia S, Benjamin RS et al (2010) Soft tissue sarcoma. J Natl Compr Canc Netw 8:630–674
Cancer. Net Editorial Board (2013) Oncologist-approved cancer information from the American Society of Clinical Oncology. http://www.cancer.net/cancer-types/sarcoma/statistics. Accessed 30 Nov 2015
Zhang CX, Lippard SJ (2003) New metal complexes as potential therapeutics. Curr Opin Chem 7:481–489
Chaudhary A, Singh AK, Singh RV (2006) Investigations of the possible pharmacological effects of organotin(II) complexes. J Inorg Biochem 100:1632–1645
Alama A, Tasso B, Novelli F, Sparatore F (2009) Organometallic compound in oncology: implications of novel organotins as antitumor agents. Drug Discov 14:500–508
Allardyce CS, Dyson PJ (2001) Ruthenium in medicine: current clinical uses and future prospects. Platin Met Rev 45:62–69
Aird RE, Cummings J, Ritchie AA et al (2002) In vitro and in vivo activity and cross resistance profiles of novel ruthenium (II) organometallic arene complexes in human ovarian cancer. Br J Cancer 86:1652–1657
Yan YK, Melchart M, Habtemariam A, Sadler PJ (2005) Organometallic chemistry, biology and medicine: ruthenium arene anticancer complexes. Chem Comm 38:4764–4776
Chelopo MP, Pawar SA, Sokhela MK et al (2013) Anticancer activity Of ruthenium(II) arene complexes bearing 1,2,3,4-tetrahydroisoquinoline amino alcohol ligands. Eur J Med Chem 66:407–414
Queiroz SL, Batista AA, Oliva G et al (1998) The reactivity of five-coordinate Ru (II)(1, 4-bis (diphenylphosphino) butane) complexes with the N-donor ligands: ammonia, pyridine, 4-substituted pyridines, 2, 2′-bipyridine, bis (o-pyridyl) amine, 1, 10-phenanthroline, 4, 7-diphenylphenanthroline and ethylenediamine. Inorg Chim Acta 267:209–221
Camargo MS, da Silva MM, Correa RS et al (2016) Inhibition of human DNA topoisomerase IB by nonmutagenic ruthenium(II)-based compounds with antitumoral activity. Metallomics 8:179–192
Blessing RH (1995) An empirical connection for absorption anisotropy. Acta Cryst A51:33–38
Enraf-Nonius (1997–2000). COLLECT. Nonius BV, Delft, The Netherlands
Otwinowski Z, Minor W (1997) Macromolecular crystallography, part A. Methods in enzymology. Academic Press, New York, pp 307–326
Sheldrick M (1997) SHELXS-97. Program for crystal structure resolution. University of Göttingen, Göttingen
Sheldrick M (1997) SHELXL-97. Program for crystal structures analysis. University of Göttingen, Göttingen
Farrugia LJ (1997) ORTEP-3 for Windows—A version of ORTEP-III with a graphical user interface (GUI). J Appl Cryst 30:565–566
Lima AP, Pereira FC, Vilanova-Costa A et al (2010) The ruthenium complex cis-(dichloro)tetrammineruthenium(III) chloride induces apoptosis and damages DNA in murine sarcoma 180 cells. J Biosci 35:371–378
Silveira-Lacerda EP, Vilanova-Costa A, Hamaguchi A et al (2010) The ruthenium complex cis-(Dichloro)tetraammineruthenium(III) chloride presents selective cytotoxicity against murine B cell lymphoma (A-20), murine ascitic sarcoma 180 (S-180), human breast adenocarcinoma (SK-BR-3), and human T cell leukemia (Jurkat) tumor cell lines. Biol Trace Elem Res 135:98–111
Mosman T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 16:55–63
Singh NP, McCoy MT, Tice RR, Schneider EL (1998) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191
Kobayashi H, Sugiyama C, Morikawa Y et al (1995) A comparison between manual microscopic analysis and computerized image analysis in the single cell gel electrophoresis assay. MMS Commun 3:103–115
Pereira FC, Lima BA, De Lima AP et al (2015) Cis-[RuCl(BzCN)(N–N)(P–P)]PF6 complexes: synthesis and in vitro antitumor activity: (BzCN = benzonitrile; N-N = 2,2′-bipyridine; 1,10-phenanthroline; P–P = 1,4-bis(diphenylphosphino) butane, 1,2-bis(diphenylphosphino)ethane, or 1,1′-(diphenylphosphino)ferrocene). J Inorg Biochem 149:91–101
Correa RS, de Oliveira KM, Delolo FG et al (2015) Ru(II)-based complexes with N-(acyl)-N′, N′-(disubstituted)thiourea ligands: synthesis, characterization, BSA- and DNA-binding studies of new cytotoxic agents against lung and prostate tumour cells. J Inorg Biochem 150:63–71
Mahavorasirikul W, Viyanant V, Chaijaroenkul W et al (2010) Cytotoxic activity of Thai medicinal plants against human cholangiocarcinoma, laryngeal and hepatocarcinoma cells in vitro. BMC Complement Altern Med 28:10–55
Pompilho WM, Borges FB, Miguel EC (2013) Biotechnology and biodiversity: the Brazilian forests as source of new drugs with antitumor properties. Acta Scientiae Tecncae 1:22–35
Chen Y, Qin MY, Wu LW et al (2013) Synthesis, characterization, and anticancer activity of ruthenium(II)-β-carboline complex. Eur J Med Chem 70:120–129. doi:10.1016/j.ejmech.2013.09.051
Almeida VL, Leitão A, ReinaI L et al (2005) Cancer and cell cycle-specific and cell cycle nonspecific anticancer DNA-interactive agents: an introduction. Quím. Nova 28:118–129
Galluzzi L, Vitale I, Abrams JM et al (2012) Molecular definitions of cell death subroutines: recommendations of the nomenclature committee on cell death. Cell Death Differ 19:107–120
Kasper C, Alborzinia H, Can S et al (2012) Synthesis and cellular impact of diene–ruthenium(II) complexes: a new class of organoruthenium anticancer agents. J Inorg Biochem 106:126–133
Yang X, Chen L, Liu Y et al (2012) Ruthenium methylimidazole complexes induced apoptosis in lung cancer A549 cells through intrinsic mitochondrial pathway. Biochimie 94:345–353
Lugli E, Troiano L, Ferraresi R et al (2005) Characterization of cells with different mitochondrial membrane potential during apoptosis. Cytometry A 68:28–35
Marverti G, Ligabue A, Montanari M et al (2011) Characterization of the cell growth inhibitory effects of a novel DNA-intercalating bipyridyl-thiourea-Pt(II) complex in cisplatin-sensitive and -resistant human ovarian cancer cells. Invest New Drugs 29:73–86
Qian C, Wang JQ, Song CL et al (2013) The induction of mitochondria-mediated apoptosis in cancer cells by ruthenium(II) asymmetric complexes. Metallomics 5:844–854
Liebermann DA, Hoffman B, Steinman RA (1995) Molecular controls of growth arrest and apoptosis: p53-dependent and independent pathways. Oncogene 11:199–210
Vousden KH, Lu X (2002) Live or let die: the cell’s response to p53. Nat Rev Cancer 2:594–604
Li L, Wong YS, Chen T (2012) Ruthenium complexes containing bis-benzimidazole derivatives as a new class of apoptosis inducers. Dalton Trans 41:1138–1141
Xie YY, Li ZZ, Lin GJ et al (2013) DNA interaction, cytotoxicity, apoptotic activity, cell cycle arrest, reactive oxygen species and mitochondrial membrane potential assay induced by ruthenium(II) polypyridyl complexes. Inorg Chim Acta 405:228–234
Sampath K, Sathiyaraj S, Raja G, Jayabalakrishnan C (2013) Mixed ligand ruthenium(III) complexes of benzaldehyde 4-methyl-3-thiosemicarbazones with triphenylphosphine/triphenylarsine co-ligands: synthesis, DNA binding, DNA cleavage, antioxidative and cytotoxicity activity. J Mol Struct 105:582–592
Wang JQ, Zhang PY, Qian C (2014) Mitochondria are the primary target in the induction of apoptosis by chiral ruthenium(II) polypyridyl complexes in cancer cells. J Biol Inorg Chem 19:335–348
Hsu CC, Tseng LM, Lee HC (2016) Role of mitochondrial dysfunction in cancer progression. Exp Biol Med 241(12):1281–1295
Galluzzi L, Bravo-San PJM, Vitale I et al (2015) Essential versus accessory aspects of cell death: recommendations of the NCCD. Cell Death Differ 22:58–73
Kroemer G (1998) The mitochondrion as an integrator/coordinator of cell death pathways. Cell Death Differ 5:547–548
Castedo M, Ferri K, Roumier T, Metivier D, Zamzami N, Kroemer G (2002) Quantitation of mitochondrial alterations associated with apoptosis. J Immunol Methods 265:39–47
Xiaofei E, Kowalik TF (2014) The DNA damage response induced by infection with human cytomegalovirus and other viroses. Viruses 6:2155–2185
Fridman JS, Lowe SW (2003) Control of apoptosis by p53. Oncogene 22:9030–9040
Olive PL, Banáth JP (2006) The comet assay: a method to measure DNA damage in individual cells. Nat Protoc 1:23–29
Acknowledgements
The authors gratefully acknowledge the financial support from Research and Project Financing (FINEP) (Grant No. 01.06.0941.00/CT-Saúde to Dr. Elisângela de Paula Silveira-Lacerda), the São Paulo Research Foundation (FAPESP) (Process 2014/10516-7) and from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. They would also like to thank the Coordination for the Improvement of Higher Education Personnel (CAPES) for the fellowship to Wanessa Carvalho Pires.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11010_2017_3129_MOESM1_ESM.doc
Supplementary crystallographic data for compounds [Ru(Spy)(bipy)(dppe)]PF6 (1), [Ru(Spy)(bipy)(dppp)]PF6 (2) and [Ru(Spy)(bipy)(dppf)]PF6 (4) complexes (CCDC 1427219, 1427220 and 1427801, respectively) can be obtained free of charge via http://www.cdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Centre (or see this session), 12 Union Road, Cambridge CB2 1 EZ, UK; fax: +44 1223 336 033; or e-mail: deposit@ccdc.com.ac.uk. Supplementary material 1 (DOC 517 kb)
Rights and permissions
About this article
Cite this article
Pires, W.C., Lima, B.A.V., de Castro Pereira, F. et al. Ru(II)/diphenylphosphine/pyridine-6-thiolate complexes induce S-180 cell apoptosis through intrinsic mitochondrial pathway involving inhibition of Bcl-2 and p53/Bax activation. Mol Cell Biochem 438, 199–217 (2018). https://doi.org/10.1007/s11010-017-3129-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-017-3129-3