Abstract
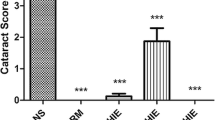
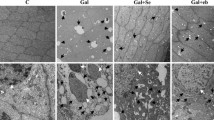
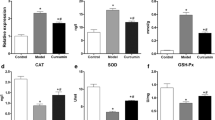
Selenite cataracts are effective and convenient animal models for simulation of human senile nuclear cataracts. These models are widely used to study the effects of various stresses on eye lenses and to screen anticataract drugs. However, there have been no comprehensive toxicological evaluations of these animal models. To investigate the effects of sodium selenite on some important organs in selenite cataract model animals, this study analyzed (1) histopathology by hematoxylin and eosin (H&E) staining; (2) methionine sulfoxide reductase (Msr) A and B1 protein expression; (3) glutathione peroxidase (GPx), thioredoxin reductase (TrxR) and superoxide dismutase (SOD) activity; and (4) malondialdehyde (MDA) levels in the liver, kidney, and brain in a selenite cataract rat model. The results showed that sodium selenite induced severe oxidative damage, especially in the hippocampus and corpus striatum of the brain, in Sprague–Dawley (SD) rats. This damage was evidenced by mild gliocyte proliferation, significant disorder of neuronal arrangement with acidophilic changes in the hippocampus, and significant occurrence of focal microglia or lymphocytic infiltration in the corpus striatum after selenite injection for cataract simulation. The damage was closely related to significant decreases in antioxidant enzyme expression and activity and significant increases in lipid peroxidation (MDA) levels. Furthermore, nonsignificant swelling and scattered spotty necrosis were observed in the liver. These results imply that physiological changes in model animals should be considered when carrying out anticataract drug screening and that pathological changes in other nontarget organs should be prevented.






Similar content being viewed by others
References
Tolbert WD, Subedi GP, Gohain N, Lewis GK, Patel KR, Barb AW et al (2019) From rhesus macaque to human: structural evolutionary pathways for immunoglobulin G subclasses. mAbs 11:709–724. https://doi.org/10.1080/19420862.2019.1589852
Swearengen JR (2018) Choosing the right animal model for infectious disease research. Animal Model Exp Med 1:100–108. https://doi.org/10.1002/ame2.12020
Xiaoguang W, Jianjun C, Qinying C, Hui Z, Lukun Y, Yazhen S (2018) Establishment of a Valuable Mimic of Alzheimer's Disease in Rat Animal Model by Intracerebroventricular Injection of Composited Amyloid Beta Protein. J Vis Exp:56157. https://doi.org/10.3791/56157
World Health Organization (2018) Priority eye diseases. In: Blindness and vision impairment prevention. https://www.who.int/blindness/causes/priority/en/index1.html. Accessed 18 Apr 2018
Khan SA, Choudhary R, Singh A, Bodakhe SH (2016) Hypertension potentiates cataractogenesis in rat eye through modulation of oxidative stress and electrolyte homeostasis. J Curr Ophthalmol 28:123–130. https://doi.org/10.1016/j.joco.2016.05.001
Shearer TR, Ma H, Fukiage C, Azuma M (1997) Selenite nuclear cataract: review of the model. Mol Vis 3:8
Kyselova Z (2010) Different experimental approaches in modelling cataractogenesis: an overview of selenite-induced nuclear cataract in rats. Interdiscip Toxicol 3:3–14
Ma Y, Wu M, Li D, Li XQ, Li P, Zhao J, Luo MN, Guo CL, Gao XB, Lu CL, Ma X (2012) Embryonic developmental toxicity of selenite in zebrafish (Danio rerio) and prevention with folic acid. Food Chem Toxicol 50:2854–2863. https://doi.org/10.1016/j.fct.2012.04.037
Melčová M, Száková J, Mlejnek P, Zídek V, Fučíková A, Praus L, Zídková J, Mestek O, Kaňa A, Mikulík K, Tlustoš P (2018) The effect of zinc and/or vitamin E supplementation on biochemical parameters of selenium-overdosed rats. Pol J Vet Sci 21:731–740
Lohr JW, Willsky GR, Acara MA (1998) Renal Drug Metabolism. Pharmacol Rev 50:107–141
Babizhayev MA (2016) Generation of reactive oxygen species in the anterior eye segment. Synergistic codrugs of N-acetylcarnosine lubricant eye drops and mitochondria-targeted antioxidant act as a powerful therapeutic platform for the treatment of cataracts and primary open-angle glaucoma. BBA Clin 6:49–68. https://doi.org/10.1016/j.bbacli.2016.04.004
Chen H, Tian W, Huang K (2013) Effect of blood-retinal barrier development on formation of selenite nuclear cataract in rat. Toxicol Lett 216:181–188. https://doi.org/10.1016/j.toxlet.2012.11.016
Hafeman DG, Sunde RA, Hoekstra WG (1974) Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. J Nutr 104:580–587. https://doi.org/10.1093/jn/104.5.580
Brandstaedter C, Fritz-Wolf K, Weder S, Fischer M, Hecker B, Rahlfs S, Becker K (2018) Kinetic characterization of wild-type and mutant human thioredoxin glutathione reductase defines its reaction and regulatory mechanisms. FEBS J 285:542–558. https://doi.org/10.1111/febs.14357
Chu S, Wang L, Mao X, Peng W (2016) Improvement of Huangqi decoction on endothelial dysfunction in 5/6 Nephrectomized rats. Cell Physiol Biochem 40:1354–1366. https://doi.org/10.1159/000453188
Zeng J, Zhou J, Huang K (2009) Effect of selenium on pancreatic proinflammatory cytokines in streptozotocin-induced diabetic mice. J Nutr Biochem 20:530–536. https://doi.org/10.1016/j.jnutbio.2008.05.012
Das JK, Sarkar S, Hossain SU, Chakraborty P, Das RK, Bhattacharya S (2013) Diphenylmethyl selenocyanate attenuates malachite green induced oxidative injury through antioxidation & inhibition of DNA damage in mice. Indian J Med Res 137:1163–1173
Boujbiha MA, Hamden K, Guermazi F, Bouslama A, Omezzine A, Kammoun A, el Feki A (2009) Testicular toxicity in mercuric chloride treated rats: association with oxidative stress. Reprod Toxicol 28:81–89. https://doi.org/10.1016/j.reprotox.2009.03.011
Ray G, Husain SA (2002) Oxidants, antioxidants and carcinogenesis. Indian J Exp Biol 40:1213–1232
Kim HY, Gladyshev VN (2007) Methionine sulfoxide reductases: selenoprotein forms and roles in antioxidant protein repair in mammals. Biochem J 407:321–329
David LL, Shearer TR (1984) State of sulfhydryl in selenite cataract. Toxicol Appl Pharmacol 74:109–115. https://doi.org/10.1016/0041-008X(84)90276-X
Qin S, Huang K, Gao J, Huang D, Cai T, Pan C (2009) Comparison of glutathione peroxidase 1 and iodothyronine deiodinase 1 mRNA expression in murine liver after feeding selenite or selenized yeast. J Trace Elem Med Biol 23:29–35. https://doi.org/10.1016/j.jtemb.2008.11.001
Ahmadvand H, Ghasemi Dehnoo M, Cheraghi R, Rasoulian B, Ezatpour B, Azadpour M, Baharvand K (2014) Amelioration of altered serum, liver, and kidney antioxidant enzymes activities by sodium selenite in alloxan-induced diabetic rats. Rep Biochem Mol Biol 3:14–20
Thirunavukkarasu C, Premkumar K, Sheriff AK, Sakthisekaran D (2008) Sodium selenite enhances glutathione peroxidase activity and DNA strand breaks in hepatoma induced by N-nitrosodiethylamine and promoted by phenobarbital. Mol Cell Biochem 310:129–139. https://doi.org/10.1007/s11010-007-9673-5
Dougherty JJ, Hoekstra WG (1982) Stimulation of lipid peroxidation in vivo by injected selenite and lack of stimulation by selenate. Proc Soc Exp Biol Med 169:209–215
Moskovitz J, Bar-Noy S, Williams WM, Requena J, Berlett BS, Stadtman ER (2001) Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc Natl Acad Sci U S A 98:12920–12925. https://doi.org/10.1073/pnas.231472998
Ruan H, Tang XD, Chen ML, Joiner ML, Sun G, Brot N, Weissbach H, Heinemann SH, Iverson L, Wu CF, Hoshi T (2002) High-quality life extension by the enzyme peptide methionine sulfoxide reductase. Proc Natl Acad Sci U S A 99:2748–2753. https://doi.org/10.1073/pnas.032671199
Liang X, Fomenko DE, Hua D, Kaya A, Gladyshev VN (2010) Diversity of protein and mRNA forms of mammalian methionine sulfoxide reductase B1 due to intronization and protein processing. PLoS One 5:e11497. https://doi.org/10.1371/journal.pone.0011497
Kumari RP, Anbarasu K (2014) Protective role of C-phycocyanin against secondary changes during sodium selenite mediated cataractogenesis. Nat Prod Bioprospect 4:81–89. https://doi.org/10.1007/s13659-014-0008-4
Ojeda ML, Nogales F, Vázquez B, Delgado MJ, Murillo ML, Carreras O (2009) Alcohol, gestation and breastfeeding: selenium as an antioxidant therapy. Alcohol Alcohol 44:272–277. https://doi.org/10.1093/alcalc/agp004
Shilo S, Pardo M, Aharoni-Simon M, Glibter S, Tirosh O (2008) Selenium supplementation increases liver MnSOD expression: molecular mechanism for hepato-protection. J Inorg Biochem 102:110–118. https://doi.org/10.1016/j.jinorgbio.2007.07.027
Orhan H, Marol S, Hepsen IF, Sahin G (1999) Effects of some probable antioxidants on selenite-induced cataract formation and oxidative stress-related parameters in rats. Toxicology 139:219–232. https://doi.org/10.1016/S0300-483X(99)00128-6
Li N, Zhu Y, Deng X, Gao Y, Zhu Y, He M (2011) Protective effects and mechanism of tetramethylpyrazine against lens opacification induced by sodium selenite in rats. Exp Eye Res 93:98–102. https://doi.org/10.1016/j.exer.2011.05.001
Musik I, Kiełczykowska M, Kocot J (2013) Oxidant balance in brain of rats receiving different compounds of selenium. Biometals 26:763–771. https://doi.org/10.1007/s10534-013-9654-y
Acknowledgments
The authors wish to thank Prof. Kaixun Huang of Huazhong University of Science and Technology, P. R. China for his suggestion in the biochemical analysis.
Funding
This work was supported by grants from “Opening fund of Hubei Key Laboratory of Bioinorganic Chemistry & Materia Medica (No. BCMM201703)” and “Ph.D. research fund of Wuhan Technology and Business University (No. D2015004)”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All animal experiments complied with the ARRIVE guidelines and were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 8023, revised 1978). All experimental procedures involving animals were approved by Scientific Research Department of Wuhan Technology and Business University.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Significant pathological changes occurred in the hippocampus and corpus striatum in the brain in cataract model rats.
• GPx, SOD, and MsrA levels were significantly decreased in the brains of selenite cataract model rats.
• Decreases in GPx, SOD, and MsrA levels and increases in MDA levels caused pathological changes.
• Pathological changes should be taken seriously during anticataract drug screening.
Electronic supplementary material
ESM 1
(PDF 1099 kb)
Rights and permissions
About this article
Cite this article
Chen, H., Zhou, J. Effects of Sodium Selenite on Oxidative Damage in the Liver, Kidney and Brain in a Selenite Cataract Rat Model. Biol Trace Elem Res 197, 533–543 (2020). https://doi.org/10.1007/s12011-019-02000-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-02000-1