Abstract
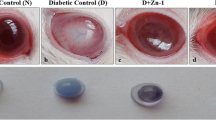
Selenite and ebselen supplementation has been shown to possess anti-cataract potential in some experimental animal models of cataract, however, the underlying mechanisms remain unclear. The present study was designed to evaluate the anti-cataract effects and the underlying mechanisms of selenite and ebselen supplementation on galactose induced cataract in rats, a common animal model of sugar cataract. Transmission electron microscopy images of lens fiber cells (LFC) and lens epithelial cells (LEC) were observed in d-galactose-induced experimental cataractous rats treated with or without selenite and ebselen, also redox homeostasis and expression of proteins such as selenoprotein R (SELR), 15kD selenoprotein (SEP15), superoxide dismutase 1 (SOD1), catalase (CAT), β-crystallin protein, aldose reductase (AR) and glucose-regulated protein 78 (GRP78) were estimated in the lenses. The results showed that d-galactose injection injured rat lens and resulted in cataract formation; however, selenite and ebselen supplementation markedly alleviated ultrastructural injury of LFC and LEC. Moreover, selenite and ebselen supplementation could mitigate the oxidative damage in rat lens and increase the protein expressions of SELR, SEP15, SOD1, CAT and β-crystallin, as well as decrease the protein expressions of AR and GRP78. Taken together, these findings for the first time reveal the anti-cataract potential of selenite and ebselen in galactosemic cataract, and provide important new insights into the anti-cataract mechanisms of selenite and ebselen in sugar cataract.








Similar content being viewed by others
Abbreviations
- AR:
-
Aldose reductase
- CAT:
-
Catalase
- DMSO:
-
Dimethyl sulfoxide
- ECL:
-
Enhanced chemiluminescence
- ER:
-
Endoplasmic reticulum
- GPX:
-
Glutathione peroxidase
- GRP:
-
Glucose-regulated protein
- LEC:
-
Lens epithelial cell
- LFC:
-
Lens fiber cell
- MDA:
-
Malondialdehyde
- MetO:
-
Methionine sulfoxide
- MSR:
-
Methionine sulfoxide reductase
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenylte-trazolium bromide
- OLB:
-
Osmiophilic lamellar bodies
- PBS:
-
Phosphate-buffered saline
- PC:
-
Protein carbonyl
- ROS:
-
Reactive oxygen species
- SELR:
-
Selenoprotein R
- SEP15:
-
15kD selenoprotein
- SOD1:
-
Superoxide dismutase 1
- TBS-T:
-
Tris-buffered saline with Tween
- TEM:
-
Transmission electron microscopy
- TSH:
-
Total mercapto group
References
Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigó R, Gladyshev VN (2003) Science 300:1439–1443
Rayman MP (2012) Lancet 379:1256–1268
Flohé L (2005) Dev Ophthalmol 38:89–102
Zhu X, Lu Y (2012) Curr Eye Res 37:163–169
Lapchak PA, Zivin JA (2003) Stroke 34(8):2013–2018
Bosch-Morell F, Romá J, Puertas FJ, Marín N, Díaz-Llopis M, Romero FJ (1999) Free Radical Biol Med 27:388–391
Aydemir O, Güler M, Kaya MK, Deniz N, Üstündağ B (2012) J Cataract Refract Surg 38(12):2160–2166
Acosta PB, Gross KC (1995) Eur J Pediatr 154:87–92
Wu DM, Lu J, Zheng YL, Zhou Z, Shan Q, Ma DF (2008) Neurobiol Learn Mem 90:19–27
Hu TS, Datiles M, Kinoshita JH (1983) Invest Ophth Vis Sci 24(5):640–644
Sakthivel M, Geraldine P, Thomas PA (2011) Graef Arch Clin Exp 249:1201–1210
Ohta Y, Torii H, Yamasaki T, Niwa T, Majima Y, Ishiguro I (1997) J Ocul Pharmacol Ther 13:537–550
Hirai T, Majima Y, Ohta Y, Ishiguro I (1986) Folia Ophthal Jpn (Nippon Ganka Kiyo) 37:1266–1270
Kasaikina MV, Fomenko DE, Labunskyy VM, Lachke SA, Qiu W, Moncaster JA, Zhang J, Wojnarowicz MW Jr, Natarajan SK, Malinouski M, Schweizer U, Tsuji PA, Carlson BA, Maas RL, Lou MF, Goldstein LE, Hatfield DL, Gladyshev VN (2011) J Biol Chem 286:33203–33212
Marchetti MA, Pizarro GO, Sagher D, DeAmicis C, Brot N, Hejtmancik JF, Weissbach H, Kantorow M (2005) Invest Ophth Vis Sci 46:2107–2112
Freel CD, Gilliland KO, Wesley Lane C, Giblin FJ, Joseph Costello M (2002) Exp Eye Res 74:689–702
De Luca A, Sacchetta P, Nieddu M, Di Ilio C, Favaloro B (2007) BMC Mol Biol 8:39–49
Hafeman DG, Sunde RA, Hoekstra WG (1974) J Nutr 104:580–587
Cao G, Cutler RG (1995) Arch Biochem Biophys 320:106–114
Ceriello A, Bortolotti N, Crescentini A, Motz E, Lizzio S, Russo A, Ezsol Z, Tonutti L, Taboga C (1998) Eur J Clin Invest 28:329–333
Andley UP (2008) Int J Biochem Cell Biol 40(3):317–323
Cheng HM, Gonzalez RG (1986) Metabolism 35:10–14
Lee AS (2001) Trends Biochem Sci 26:504–510
McCarthy CA, Taylor HR (1996) Invest Ophthalmol Vis Sci 37:1720–1723
Naziroglu M, Karaoğlu A, Aksoy AO (2004) Toxicology 195(2–3):221–230
Kador PF, Inoue J, Secchi EF, Lizak MJ, Rodriguez L, Mori K, Greentree W, Blessing K, Lackner PA, Sato S (1998) Exp Eye Res 67(2):203–208
Peek R, McAvoy JW, Lubsen NH, Schoenmakers JG (1992) Dev Biol 152(1):152–160
Spector A (1995) FASEB J 9:1173–1182
Dai J, Liu H, Zhou J, Huang K (2016) Int J Mol Sci 17(2):E231
Ou Y, Liao G, Yuan Z, Wu W (2012) Curr Eye Res 37(3):187–194
Orrenius S (2007) Drug Metab Rev 39:443–455
Shimizu N, Hosogi N, Hyon G, Jiang S, Inoue K, Park P (2006) J Gen Plant Pathol 72:6–15
Wang W, Xia MX, Chen J, Yuan R, Deng FN, Shen FF (2016) Biochemistry (Mosc) 81(5):465–480
Chul-Ho J, Sang HJ (2016) J Cancer Prev 21(1):13–20
Lou MF (2003) Prog Retin Eye Res 22(5):657–682
Steinbrenner H, Sies H (2009) Biochim Biophys Acta 1790(11):1478–1485
Reeves MA, Hoffmann PR (2009) Cell Mol Life Sci 66:2457–2478
Xu A, Prophete C, Chen LC, Emala CW, Cohen MD (2011) J Toxicol Env Health 74:887–902
Kim HY, Gladyshev VN (2007) Biochem J 407:321–329
Li Y, Zhang W, Li P, Huang KX (2011) Exp Eye Res 92:401–407
Gladyshev VN, Jeang KT, Wootton JC, Hatfield DL (1998) J Biol Chem 273:8910–8915
Korotkov KV, Kumaraswamy E, Zhou Y, Hatfield DL, Gladyshev VN (2001) J Biol Chem 276:15330–15336
Labunskyy VM, Yoo MH, Hatfield DL, Gladyshev VN (2009) Biochemistry 48:8458–8465
Ferguson AD, Labunskyy VM, Fomenko DE, Araç D, Chelliah Y, Amezcua CA, Rizo J, Gladyshev VN, Deisenhofer J (2006) J Biol Chem 281:3536–3543
Parnham MJ, Sies H (2013) Biochem Pharmacol 86:1248–1253
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Project No. 21271077). We give our sincere thanks to the faculties from Analytical and Testing Centre of Huazhong University of Science and Technology for their help in the bioanalysis.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dai, J., Zhou, J., Liu, H. et al. Selenite and ebselen supplementation attenuates d-galactose-induced oxidative stress and increases expression of SELR and SEP15 in rat lens. J Biol Inorg Chem 21, 1037–1046 (2016). https://doi.org/10.1007/s00775-016-1400-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-016-1400-9