Abstract
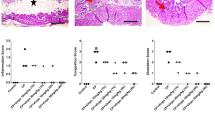
This study aims to investigate protective effects of boron against cyclophosphamide-induced bladder toxicity that produces oxidative stress and leads to apoptosis of the cells. In total, 24 rats were divided into 4 equal groups. The control group received saline. The 2nd experimental group received 200 mg kg of cyclophosphamide i.p. on the 4th day while the 3rd group was given only boron (200 mg kg, i.p.) for 6 days. In the 4th group, boron was given for 6 days and cyclophosphamide (200 mg kg, i.p.) was administrated on the 4th day. Twenty-four hours after the last boron or cyclophosphamide administration, rats were sacrificed under anesthesia. Bladder tissues of rats were taken for histological and immunohistochemical (apoptotic markers such as caspase-3, bcl-2, and bax) and blood was taken for the biochemical (serum total thiol, serum natural thiol, serum thiol-disulfide) analysis. Transient epithelial thinning, edema, marked inflammatory reaction, and bleeding were observed in bladders of the group that received cyclophosphamide. Also, the activity of bax and caspase-3-positive cells increased while the number of bcl-2-positive cells decreased. In the same group, serum natural thiol and total thiol levels decreased while serum disulfide levels increased, which indicates oxidative stress. On the other hand, in the boron+cyclophosphamide group pretreatment with boron protected, the bladder tissue and the number of bcl-2-positive cells increased, and bax and caspase-3-positive cells decreased, showing antiapoptotic effects of boron against cyclophosphamide-induced toxicity. In parallel with the findings of this group, native thiol and total thiol levels increased and serum disulfide levels decreased pointing out to a decreased oxidative stress. Our results indicate that boron pretreatment significantly protects rat bladder against cyclophosphamide-induced bladder damage due to its antiapoptotic and antioxidant properties.





Similar content being viewed by others
References
Dollery C (1999) Therapeutic drugs. Edinburgh, Churchill Livingstone
Abraham P, Isaac B, Ramamoorthy H, Natarajan K (2011) Oral glutamine attenuates cyclophosphamide-induced oxidative stress in the bladder but does not prevent hemorrhagic cystitis in rats. J Med Toxicol 7(2):118–124
Rang HP, Dale MM, Ritter JM, Flower RJ, Henderson G (2012) Antidepressant drugs. In: Humphrey P, Rang Maureen M, Dale James M, Ritter Rod J, Henderson FG (eds) Rang and Dale’s pharmacology, 7th edn. Elsevier/Churchill Livingstone, Edinburgh, pp 564–583
Erel O, Neselioglu S (2014) A novel and automated assay for thiol/disulfide homeostasis. Clin Biochem 47(18):326–332
Murray FJ (1998) A comparative review of the pharmacokinetics of boric acid in rodents and humans. Biol Trace Elem Res 66:331–341
Hunt CD (1994) The biochemical effects of physiologic amounts of dietary boron in animal nutrition models. Environ Health Perspect 102(Suppl 7):35–43
Nielsen FH, Hunt CD, Mullen LM, Hunt JR (1987) Effect of dietary boron on mineral, estrogen, and testosterone metabolism in postmenopausal women. FASEB J 1:394–397
Meacham SL, Taper LJ, Volpe SL (1994) Effects of boron supplementation on bone mineral density and dietary, blood, and urinary calcium, phosphorus, magnesium, and boron in female athletes. Environ Health Perspect 102:79–82
Turkez H, Geyikoglu F, Tatar A, Keles S, Ozkan A (2007) Effects of some boron compounds on peripheral human blood. Z Naturforsch 62:889–896
Ince S, Kucukkurt I, Cigerci IH, Fatih Fidan A, Eryavuz A (2010) The effects of dietary boric acid and borax supplementation on lipid peroxidation, antioxidant activity, and DNA damage in rats. J Trace Elem Med Biol 24:161–164
Pfeiffer CC, Hallman LF, Gersh I (1945) Boric acid ointment: a study of possible intoxication in the treatment of burns. J Am Med Assoc 128:266–274
Erel O (2005) A new automated colorimetric method for measuring total oxidant status. Clin Biochem 38:1103–1111
Ayhanci A, Cengiz M, Kutlu HM, Vejselova D (2016) Protective effects of ellagic acid in D-galactosamine-induced kidney damage in rats. Cytotechnology 68:1763–1770
Davis L, Kuttan G (2000) Effect of Withania somnifera on cyclophosphamide-induced urotoxicity. Cancer Lett 148:9–17
Manesh C, Kuttan G (2005) Effect of naturally occurring isothiocyanates in the inhibition of cyclophosphamide-induced urotoxicity. Phytomedicine 12:487–493
Beyer-Boon ME, De Voogt HJ, Shaberg A (1978) The effects of cyclophosphamide treatment on the epithelium and stroma of the urinary bladder. Eur J Cancer 14:1029–1035
Gray KJ, Engelmann UH, Johnson EH, Fishman IJ (1986) Evaluation of misoprostol cytoprotection of the bladder with cyclophosphamide (Cytoxan) therapy. J Urol 136(2):497–500
Mythili Y, Sudharsan PT, Selvakumar E, Varalakshmi P (2004) Protective effect of DL-a-lipoic acid on cyclophosphamide induced oxidative cardiac injury. Chem Biol Interact 151:13–19
Arumugam N, Sivakumar V, Thanislass J, Devaraj H (1997) Effects of acrolein on rat liver antioxidant defense system. Indian J Exp Biol 35:1373–1374
McDermott EM, Powell RJ (1996) Incidence of ovarian failure in systemic lupus erythematosus after treatment with pulse cyclophosphamide. Ann Rheum Dis 55:224–229
Liu B, Chen Y, St Clair DK (2008) ROS and p53: a versatile partnership. Free Radic Biol Med 44:1529–1535
Chaudhari M, Jayaraj R, Bhaskar AS, Lakshmana Rao PV (2009) Oxidative stress induction by T-2 toxin causes DNA damage and triggers apoptosis via caspase pathway in human cervical cancer cells. Toxicology 262:153–161
Frenzel A, Grespi F, Chmelewskij W, Villunger A (2009) Bcl2 family proteins in carcinogenesis and the treatment of cancer. Apoptosis 14:584–596
Kanchan B, Firoz A, Hina R, Sheikh R (2008) Protective effect of S-allylcysteine against cyclophosphamide-induced bladder hemorrhagic cystitis in mice. Food Chem Toxicol 46:3368–3374
Sherif IO (2018) Uroprotective mechanism of quercetin against cyclophosphamide-induced urotoxicity: effect on oxidative stress and inflammatory markers. J Cell Biochem 119:7441–7448
Erdoğan O, Keles H (2019) Pathologic examination of the protective effect of glycyrrhizin on cyclophosphamide-induced hemorrhagic cystitis in rats. Kocatepe Vet J 12(1):89–96 l
Gelen V, Sengul E, Yıldırım S, Celebi F, Cınar A (2018) Effects of rutin on bladder contractility and histopathology in cyclophosphamide-induced hemorrhagic cystitis in rats. Ataturk University J Vet Sci 13(3):337–346.
Ergin M, Cendek BD, Neselioglu S, Avsar AF, Erel O (2015) Dynamic thiol-disulfide homeostasis in hyperemesis gravidarum. J Perinatol 35(10):788–792
Korkmaz V, Kurdoglu Z, Alisik M, Cetin O, Korkmaz H, Surer H, Erel O (2016) Impairment of thiol-disulfide homeostasis in preeclampsia. J Matern Fetal Neonatal Med 29(23):3848–3853
Arıkan YM, Toklu Y, Altınkaynak H, Tanrıverdi B, Ergin M, Biçer C (2016) A novel tool for the assessment oxidative stress in age-related macular degeneration: thiol/disulfide homeostasis revisited. Curr Eye Res 41(12):1584–1589
Bektas H, Vural G, Gumusyayla S, Deniz O, Alisik M, Erel O (2016) Dynamic thiol–disulfide homeostasis in acute ischemic stroke patients. Acta Neurol Belg 116(4):489–494
Durak D (2016) Akut bı̇lı̇er pankreatı̇t hastalarında tı̇yol dı̇sülfı̇t dengesı̇nı̇n prognostı̇k faktörler ı̇le karşılaştırılması, Uzmanlık Tezi. Yıldırım Beyazıt Üniversitesi, Ankara
Pawa S, Ali S (2006) Boron ameliorates fulminant hepatic failure by counteracting the changes associated with the oxidative stress. Chem Biol Interact 160:89–98
Ince S, Keles H, Erdogan M, Hazman O, Kucukkurt I (2012) Protective effect of boric acid against carbon tetrachloride-induced hepatotoxicity in mice. Drug Chem Toxicol 35(3):285–292
Henderson K, Stella SL, Kobylewski S, Eckhert CD (2009) Receptor activated Ca(2+) release is inhibited by boric acid in prostate cancer cells. PLoS One 4(6):e6009
Sogut I, Oglakci A, Kartkaya K, Ol KK, Sogut MS, Kanbak G, Inal ME (2015) Effect of boric acid on oxidative stress in rats with fetal alcohol syndrome. Exp Ther Med 9(3):1023–1027
Sogut I, Paltun SO, Tuncdemir M, Ersoz M, Hurdag C (2018) The antioxidant and antiapoptotic effect of boric acid on hepatoxicity in chronic alcohol-fed rats. CanJ Physiol Pharmacol 96(4):404–411
Cengiz M, Yildiz SC, Demir C, Sahin IK, Teksoy O, Ayhanci A (2019) Hepato-preventive and anti-apoptotic role of boric acid against liver injury induced by cyclophosphamide. J Trace Elem Med Biol 53:1–7
Coban FK, Liman R, Cigerci IH, Ince S, Hazman O, Bozkurt MF (2015) The antioxidant effect of boron on oxidative stress and DNA 453 damage in diabetic rats. Fresenius Environmental Bulletin 24(11)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ayhanci, A., Tanriverdi, D.T., Sahinturk, V. et al. Protective Effects of Boron on Cyclophosphamide-Induced Bladder Damage and Oxidative Stress in Rats. Biol Trace Elem Res 197, 184–191 (2020). https://doi.org/10.1007/s12011-019-01969-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01969-z