Abstract
This report explains the employing of a combination test of traditional cell culture with a quantitative real-time PCR for assessment of the antiviral effect of zinc sulfate (ZnSO4) on herpes simplex virus (HSV)-infected Vero cells. Our evidence showed that the treatment with 0.3 mM ZnSO4 strongly inhibited the replication of virus progeny (MOI 0.001) at least 68-fold less. On the other hand, the IC50 demonstrated that the highest activity of ZnSO4 was at the 0.23 mM concentration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Viral infection is a primary and major cause of death among the human and animal infectious diseases in the world [1]. Herpes simplex virus type-1 (HSV-1) is one of the members of the Herpesviridae family, which frequently associated with several diseases including herpetic stomatitis, recurrent herpes labialis, erythema multiform, cranial neuropathies, and oral squamous carcinoma. HSV-1 is capable of causing lesions at or near the point of entry into the body and also can establish a latent infection in sensory ganglia. Subsequently, the latency often increases the HSV pathogenicity [2].
Antiviral medications including Acyclovir, Famciclovir, and Valacyclovir are effective drugs for people suffering from HSV infections. Although these drugs suppress the recurrence rate and viral shedding through the effects on viral DNA replication, their drawbacks include renal failure, anaphylaxis, and hepatitis [3]. Also, a considerable amount of literature has been published on HSV resistance to these antiviral drugs. Therefore, an effective and novel therapeutic strategy is required.
Recent evidence suggested that the use of natural substances such as lysine, vitamin C, zinc, vitamin E, and adenosine monophosphate may be beneficial for preventing the active infections or recurrences of herpes simplex [4]. It should be noted that several findings by researchers pointed the antiviral activity of zinc salts against a variety of viruses, including human immunodeficiency virus (HIV), human influenza A virus, equine arteritis virus (EAV), vaccinia virus, severe acute respiratory syndrome coronavirus (SARS CoV), rhinovirus, and respiratory syncytial virus (RSV). Interestingly, zinc ions are used to treat these viral infections due to the hindrance of virus entry, a barrier of polyprotein processing or blocking the viral RNA-dependent RNA polymerase (RdRP) activity [5].
The antiviral effects of zinc ion on the HSV have been investigated by many researchers since 1967 [6]. Fridlender et al. in 1978 published a paper in which they described that the zinc ions reduced the activity of HSV DNA polymerase at a lower concentration than the cellular DNA polymerase. Furthermore, in vitro studies confirmed that 0.2 mM ZnSO4 significantly inhibited the HSV DNA replication by blocking the function of viral DNA polymerase and thus preventing the synthesis of viral proteins [7]. Also, other studies reported that zinc ions could interfere with the proper function of viral surface glycoproteins, resulting in blockage of virion penetration into the cells [6, 8].
To date, there is no study that focuses on the antiviral effect of ZnSO4 against HSV1-infected Vero cells based on the relative quantitative polymerase chain reaction (qRT-PCR) assay. QRT-PCR can provide a specific and sensitive method for determining the dynamics of virus proliferation and monitoring the treatment response.
Material and Method
Virus Culture
African green monkey kidney cells (Vero cells) (Razi Vaccine and Serum Research Institute, Karaj, Iran) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco, USA) and 100 IU/ml penicillin and 100 μg/ml streptomycin (Sigma-Aldrich, USA) in 37 °C in a humidified atmosphere containing 5% CO2 for 48 h.
Confluent monolayer cells in 75-cm2 tissue culture flasks were infected by laboratory-adapted HSV-1 (KOS strain) at 0.01 and 0.001 multiplicity of infection (MOI). The flasks were incubated for 72 h at 37 °C and HSV-1 infected cells were harvested through three freeze-thaw cycles and stored at − 70 °C.
Plaque Assay
To calculate the MOI, Vero cells were cultured in a six-well plate (SPL, Korea) followed by incubation at 37 °C with 5% CO2 for 24 h. Then, 0.5 ml of different sixfold virus dilution (10−2 to 10−6) were inoculated to monolayer cells at 37 °C. After 1 h for virus adsorption, the inoculum was removed and cells were washed twice with phosphate-buffered saline (PBS) and covered with 3 ml of overlay media containing the 2× DMEM (comprising of 4.5 g/L D-glucose without L-glutamine) (Sigma-Aldrich, USA) with 1.5% agarose gel at 1:1 (2× DMEM:agarose gel) ratio. The plate was incubated at 37 °C in 5% CO2 for 72 h. After plaque formation, second agar overlay containing neutral red was added to visualize the plaques with the naked eye and the plate was incubated at 37 °C in 5% CO2.
Cytotoxicity Test by MTT Assay
To evaluate the ZnSO4 cytotoxicity, the MTT assay (3-(4,5-dimethylthiazol-2-yl)-3,5-diphenyl tetrazolium bromide) (Sigma) was performed.
Vero cells were passaged (5 × 103 cells/well) in a 96-well plate (SPL, Korea) and the plate was incubated at 37 °C with 5% CO2 for 24 h. The medium was replaced with DMEM medium containing various concentrations of ZnSO4 and the plate was incubated for 72 h. The culture medium was removed and 5 mg/mL MTT in PBS was added to each well, and the plate was incubated for 4 h at 37 °C. After removal of supernatant, 50 μL of dimethyl sulfoxide (DMSO) was added to each well and the plate incubated for 20 min at 37 °C with 5% CO2. Then, the optical density (OD) was measured at 570 nm using the ELISA plate reader.
Finally, the viability of cells was calculated using the following equation:
Relative Quantitative PCR
Vero cells were grown to about 70% confluence in a 96-well plate and infected with HSV-1 (MOI: 0.01 and 0.01) and treated with ZnSO4 at various concentrations (100–300 μM). After 72 h incubation, the culture medium was collected, and total DNA extracted from medium using the high pure viral nucleic acid kit (Roche, Germany). Then, qRT-PCR was performed using QuantiTect Probe PCR Kits (Qiagen, Germany). Samples were examined in a 20-μL reaction containing 10 μL of the 2× Master mix, 0.2 μL probe, 0.4 μL (5 pmol) each of forward and reverse primers, 8 μL of nuclease-free water, and 1 μL of DNA. The PCR thermal profile consisted of 95 °C for 2 min (1 cycle), 95 °C for 30 s, and 60 °C for 15 s (35 cycles, respectively). Positive and negative controls were also included in all experiments, and each sample was analyzed in triplicate.
A 96-bp segment of gB gene was amplified using primers and probe which are listed in Table 1. The TaqMan probe was designed for HSV-1 specifically and confirmed by Primer Blast algorithms.
Results
Herpes Simplex Type 1 Toxicity Assay
Unlike to normal cell group, HSV1-infected Vero cells became rounded and also, some of them detached from the bottom of the plates. In this study, over 50% of the cells exhibited cytopathic effect (CPE) after 72 h. The HSV-1-induced CPE in Vero cells is presented in Fig. 1.
Cytotoxicity of ZnS04 to Cultured Cells
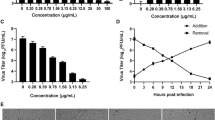
Before the determination of inhibitory concentration of ZnSO4 on HSV-1 replication, cells were exposed to the increased concentrations of ZnSO4 and the viability monitored by MTT technique as a colorimetric assay. Figure 3 implicates the cytotoxicity effect of ZnSO4 in Vero cells. According to the results, the IC50 showed the strong influence of ZnSO4 at the 0.23 mM concentration.
Evaluation of the Real-time PCR Assay
We compared the effect of ZnSO4 on relative change of HSV-1 DNA replication in treated infected cells (as a test group) and infected cells (as a control group) using qRT-PCR. To determine the level of expression, the differences of threshold cycles (∆Ct) between the test and the control group were measured. To achieve optimal relative expression results, appropriate normalization strategies were applied to reduce experimental error, and also HSV-1 (MOI: 0.001 and 0.01) was added to both test and control groups (Fig. 2a, b).
Discussion
Our interest in the zinc effect on HSV replication was due to the several previous studies on the potential activity of zinc ions against laboratory-adapted strains of HSV.
As mentioned, an early in vitro study in 1978 by Fridlender et al. showed the inhibitory effect of zinc salt on HSV-1 DNA polymerase [7]. Also, this result was gained by Gordon et al. and Shlomai et al. to describe blocking of HSV-1 DNA in vivo [9, 10]. Generally, the previous studies claimed that the zinc concentrations of 0.1 mM and 0.2 mM inhibited the production of virion progeny in HSV-infected cells. Also, zinc ions at the concentration of 0.3 mM were cytotoxic and induced the cell death. According to an investigation by Arens et al., the degree of inhibition seemed to depend on the type and strain of HSV and also the zinc salt form [8]. In another study by Kumel et al. using Electron microscopy, they showed that there was a correlation between the depositions of zinc onto the viral surface glycoproteins and inhibition of virion penetration into African green monkey kidney (AGMK) cells [6].
As shown in Fig. 2a, we found that there was a significant decline in the amount of replication of virion progeny (MOI 0.001) isolated from HSV-infected Vero cells in the presence of 0.3 mM ZnSO4. Based on the data in Fig. 3, it is apparent that ZnSO4 concentration of 0.23 mM is toxic to Vero cells. But there is no meaningful difference between 0.23 and 0.2 mM. So, we concluded that the virus inactivation was probably due to the cytotoxicity of ZnSO4 instead of interfering with adsorption and penetration processes, in contrast to the results by Kumel [6]. Also, we highlight the proposed hypothesis by Arens who concluded that several factors should be considered for inhibition of zinc-treated infected cells, such as the type of virus and cell, HSV strain, and salt form [8]. Furthermore, we showed that the MOI ratio was another important factor for the effect of zinc ions on this process.
It should be noted that we also mixed the virus and ZnSO4 and added them to Vero cells that showed similar results.
This research has thrown up many questions in need of further investigation. In addition, more studies are required on monitoring the treated infected cell by qRT-PCR technique. Also, to assess the ZnSO4 uptake, the intracellular zinc content in Vero cells should also be measured after the exposure of cells to the ZnSO4.
References
Wolfe ND, Dunavan CP, Diamond J (2007) Origins of major human infectious diseases. Nature. 447(7142):279–283
Whitley RJ, Nahmias AJ, Visintine AM, Fleming CL, Alford CA, Yeager A et al (1980) The natural history of herpes simplex virus infection of mother and newborn. Pediatrics. 66(4):489–494
Pasternak B, Hviid A (2010) Use of acyclovir, valacyclovir, and famciclovir in the first trimester of pregnancy and the risk of birth defects. JAMA 304(8):859–866
Gaby AR (2006) Natural remedies for herpes simplex. Altern Med Rev 11(2):93
Jiang Y-C, Feng H, Lin Y-C, Guo X-R (2016) New strategies against drug resistance to herpes simplex virus. Int J Oral Sci 8(1):1–6
Kümel G, Schrader S, Zentgraf H, Daus H, Brendel M (1990) The mechanism of the antiherpetic activity of zinc sulphate. J Gen Virol 71(12):2989–2997
Fridlender B, Chejanovsky N, Becker Y (1978) Selective inhibition of herpes simplex virus type 1 DNA polymerase by zinc ions. Virology 84(2):551–554
Arens M, Travis S (2000) Zinc salts inactivate clinical isolates of herpes simplex virus in vitro. J Clin Microbiol 38(5):1758–1762
Gordon YJ, Asher Y, Becker Y (1975) Irreversible inhibition of herpes simplex virus replication in BSC-1 cells by zinc ions. Antimicrob Agents Chemother 8(3):377–380
Shlomai J, Asher Y, Gordon YJ, Olshevsky U, Becker Y (1975) Effect of zinc ions on the synthesis of herpes simplex virus DNA in infected BSC-1 cells. Virology. 66(1):330–335
Acknowledgments
This work was supported by Infectious and Tropical Diseases Research Center, Health Research Institute and Department of Virology, Faculty of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fani, M., Khodadad, N., Ebrahimi, S. et al. Zinc Sulfate in Narrow Range as an In Vitro Anti-HSV-1 Assay. Biol Trace Elem Res 193, 410–413 (2020). https://doi.org/10.1007/s12011-019-01728-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01728-0