Abstract
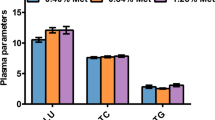
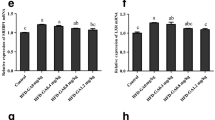
An 11-week feeding trial was carried out to investigate the effects of supplemented chromium picolinate (Cr-Pic) on the growth, whole-body composition, and relative mRNA expression related to lipogenesis and glucose metabolism in juvenile blunt snout bream. Seven isonitrogenous and isoenergetic diets with graded Cr supplementation levels were fed to triplicate groups. The final weight (FW), feed conversion ratio (FCR), and specific growth rate (SGR) were improved with increasing dietary Cr supplementation levels up to 0.4 mg/kg, and thereafter showed relatively constant. However, 12.0 mg/kg dietary Cr supplementation decreased growth and feed utilization. Based on SGR and FCR, the optimal dietary Cr supplementation level for the juvenile was estimated to be 0.28 mg/kg. Significantly higher plasma insulin levels were found in juvenile fed diets with 0.4 and 0.8 mg/kg Cr supplementation compared to those fed diet sans supplemented Cr. Plasma glucose levels decreased with increasing dietary Cr supplementation, and the lowest value was remarked in the group added 3.2 mg/kg of Cr. Adding 0.4–0.8 mg/kg Cr enhanced insulin receptor substrate 1 (IRS-1), phosphoinositide-3-kinase (PI3K), and pyruvate kinase (PK) and inhibited expression of phosphoenolpyruvate carboxykinase (PEPCK), glucose-6-phosphatase (G6Pase), and glycogen synthase (GS) mRNA levels. High dietary Cr (12.0 mg/kg) supplementation resulted in high G6Pase and PEPCK expression. The highest content of whole-body lipid was remarked in fish fed with 0.4 mg/kg dietary Cr, which related to the enhanced gene expression related to lipogenesis; thereafter, mRNA levels showed a diminishing trend. These findings indicate that optimum dietary Cr-Pic supplementation has a positive effect on growth and blood glucose homeostasis by modifying the mRNA levels related to glucose metabolism and lipogenesis in juvenile blunt snout bream.






Similar content being viewed by others
References
Vincent JB (2000) The biochemistry of chromium. J Nutr 130(4):715–718
Bona KRD, Love S, Rhodes NR, McAdory D, Sinha SH, Kern N, Kent J, Strickland J, Wilson A, Beaird J, Ramage J, Rasco JF, Vincent JB (2011) Chromium is not an essential trace element for mammals: effects of a “low- chromium” diet. J Biol Inorg Chem 16(3):381–390. https://doi.org/10.1007/s00775-010-0734-y
Anderson RA (1997) Chromium as an essential nutrient for humans. Regul Toxicol Pharmacol 26(1):35–41. https://doi.org/10.1006/rtph.1997.1136
Dallago BS, Braz S, Marçola TG, McManus C, Caldeira DF, Campeche A, Gomes EF, Paim TP, Borges BO, Louvandini H (2015) Blood parameters and toxicity of chromium picolinate oral supplementation in lambs. Biol Trace Elem Res 168(1):91–102. https://doi.org/10.1007/s12011-015-0347-y
Qiao W, Peng ZL, Wang ZS, Wei J, Zhou AG (2009) Chromium improves glucose uptake and metabolism through upregulating the mRNA levels of IR, GLUT4, GS, and UCP3 in skeletal muscle cells. Biol Trace Elem Res 131(2):133–142. https://doi.org/10.1007/s12011-009-8357-2
Sundaram B, Singhal K, Sandhir R (2012) Ameliorating effect of chromium administration on hepatic glucose metabolism in streptozotocin-induced experimental diabetes. Biofactors 38(1):59–68. https://doi.org/10.1002/biof.194
Jain SK, Rains JL, Croad JL (2007) Effect of chromium niacinate and chromium picolinate supplementation on lipid peroxidation, TNF-α, IL-6, CRP, glycated hemoglobin, triglycerides, and cholesterol levels in blood of streptozotocin-treated diabetic rats. Free Radic Biol Med 43(8):1124–1131. https://doi.org/10.1016/j.freeradbiomed.2007.05.019
NRC (National Research Council) (1997) The role of chromium in animal nutrition. National Academy, Washington, DC
Evans GW, Pouchnik DJ (1993) Composition and biological activity of chromium-pyridine carboxylate complexes. J Inorg Biochem 49(3):177–187. https://doi.org/10.1016/0162-0134(93)80003-R
Preuss HG, Echard B, Perricone NV, Bagchi D, Yasmin T, Stohs SJ (2008) Comparing metabolic effects of six different commercial trivalent chromium compounds. J Inorg Biochem 102(11):1986–1990. https://doi.org/10.1016/j.jinorgbio.2008.07.012
Wilson RP (1994) Utilization of dietary carbohydrate by fish. Aquaculture 124(1-4):67–80. https://doi.org/10.1016/0044-8486(94)90363-8
NRC (National Research Council) (2011) Nutrient requirements of fish and shrimp. National Academy, Washington, DC
Shiau SY, Chen MJ (1993) Carbohydrate utilization by tilapia Oreochromis niloticus × O.aureus was influenced by different chromium sources. J Nutr 123(10):1747–1753
Liu T, Wen H, Jiang M, Yuan DN, Gao P, Zhao YJ, Wu F, Liu W (2010) Effect of dietary chromium picolinate on growth performance and blood parameters in grass carp fingerling, Ctenopharyngodon idellus. Fish Physiol Biochem 36(3):565–572. https://doi.org/10.1007/s10695-009-9327-5
Ahmed AR, Jha AN, Davies SJ (2012) The effect of dietary organic chromium on specific growth rate, tissue chromium concentrations, enzyme activities and histology in common carp, Cyprinus carpio L. Biol Trace Elem Res 149(3):362–370. https://doi.org/10.1007/s12011-012-9436-3
Ahmed AR, Jha AN, Davies SJ (2012) The efficacy of chromium as a growth enhancer for mirror carp (Cyprinus carpio L): an integrated study using biochemical, genetic, and histological responses. Biol Trace Elem Res 148(2):187–197. https://doi.org/10.1007/s12011-012-9354-4
Wang J, Ai QH, Mai KS, Xu HG, Zuo RT (2014) Dietary chromium polynicotinate enhanced growth performance, feed utilization, and resistance to Cryptocaryon irritans in juvenile large yellow croaker (Larmichthys crocea). Aquaculture 432:321–326. https://doi.org/10.1016/j.aquaculture.2014.05.027
Ng WK, Wilson RP (1997) Chromium oxide inclusion in the diets does not affect glucose utilization and chromium retention by channel catfish, Ictalurus punctatus. J Nutr 12:2357–2362
Gatta PP, Piva A, Paolini M, Testi S, Bonaldo A, Antelli A, Mordenti A (2001) Effects of dietary organic chromium on gilthead seabream (Sparus aurata L.) performances and liver microsomal metabolism. Aquac Res 32(Suppl. 1):60–69. https://doi.org/10.1046/j.1355-557x.2001.00005.x
Selcuk Z, Tiril SU, Alagil F, Belen V, Salman M, Cenesiz S, Muglali OH, Yagci FB (2010) Effects of dietary L-carnitine and chromium picolinate supplementations on performance and some serum parameters in rainbow trout (Oncorhynchus mykiss). Aquacult Int 18(2):213–221. https://doi.org/10.1007/s10499-008-9237-z
Ministry of Agriculture of the People’s Republic of China (2016) China fishery statistical yearbook. Chinese Agricultural Press, Beijing (in Chinese)
Zhou CP, Liu B, Ge XP, Xie J, Xu P (2013) Effect of dietary carbohydrate on the growth performance, immune response, hepatic antioxidant abilities and heat shock protein 70 expression of Wuchang bream, Megalobrama amblycephala. J Appl Ichthyol 29(6):1348–1356. https://doi.org/10.1111/jai.12264
Ren MC, Habte-Tsion HM, Xie J, Liu B, Zhou QL, Ge XP, Pan LK, Chen RL (2015) Effects of dietary carbohydrate source on growth performance, diet digestibility and liver glucose enzyme activity in blunt snout bream, Megalobrama amblycephala. Aquaculture 438:75–81. https://doi.org/10.1016/j.aquaculture.2015.01.008
AOAC (Association of Official Analytical Chemists) (2003) Official methods of analysis of the association of official analytical chemists, 15th edn. Association of Official Analytical Chemists Inc, Arlington
Habte-Tsion HM, Liu B, Ren MC, Ge XP, Xie J, Zhou QL, Miao LH, Pan LK, Chen RL (2015) Dietary threonine requirement of juvenile blunt snout bream (Megalobrama amblycephala). Aquaculture 437:304–311. https://doi.org/10.1016/j.aquaculture.2014.12.018
Gao ZX, Luo W, Liu H, Zeng C, Liu XL, Yi SK, Wang WM (2012) Transcriptome analysis and SSR/SNP markers information of the blunt snout bream (Megalobrama amblycephala). PLoS One 7(8):e42637. https://doi.org/10.1371/journal.pone.0042637
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2- ∆∆CT method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Robbins KR, Norton HW, Baker DH (1979) Estimation of nutrient requirement from growth data. J Nutr 109(10):1710–1714
Mukhtar AK, Shabi FA (2013) Dietary methionine requirement of Indian major carp fry, Cirrhinus mrigala (Hamilton) based on growth, feed conversion and nitrogen retention efficiency. Aquac Res 44(2):268–281. https://doi.org/10.1111/j.1365-2109.2011.03030.x
Tacon AGJ, Beveridge MM (1982) Effects of dietary trivalent chromium on rainbow trout. Nutr Rep Int 25:49–56
Kahn BB, Flier JS (2000) Obesity and insulin resistance. J Clin Invest 106(4):473–481. https://doi.org/10.1172/JCI10842
Yang X, Palanichamy K, Ontko AC, Rao MN, Fang CX, Ren J, Sreejayan N (2005) A newly synthetic chromium complex chromium (phenylalanine) 3 improves insulin responsiveness and reduces whole body glucose tolerance. FEBS Lett 579(6):1458–1464. https://doi.org/10.1016/j.febslet.2005.01.049
Chen WY, Chen CJ, Liu CH, Mao FC (2009) Chromium supplementation enhances insulin signalling in skeletal muscle of obese KK/HlJ diabetic mice. Diabetes Obes Metab 11(4):293–303. https://doi.org/10.1111/j.1463-1326.2008.00936.x
Wang ZQ, Zhang XH, Russell JC, Hulver M, Cefalu WT (2006) Chromium picolinate enhances skeletal muscle cellular insulin signaling in vivo in obese, insulin-resistant JCR: LA-cp rats. J Nutr 136(2):415–420
Anderson RA, Polansky MM, Bryden NA, Canary JJ (1991) Supplemental chromium effects on glucose, insulin, glucagon and urinary loss in subjects consuming low chromium diets. Am J Cardiovasc Drugs 54:909–916
Hertz Y, Mader Z, Hepher B, Gertler A (1989) Glucose metabolism in the common carp (Cyprinus carpio L.): the effect of cobalt and chromium. Aquaculture 76(3-4):255–267. https://doi.org/10.1016/0044-8486(89)90079-3
Enes P, Panserate S, Kaushik S, Oliva-Teles A (2009) Nutritional regulation of hepatic glucose metabolism in fish. Fish Physiol Biochem 35(3):519–539. https://doi.org/10.1007/s10695-008-9259-5
Pereira C, Vijayan MM, Storey KB, Jones RA, Moon TW (1995) Role of glucose and insulin in regulating glycogen synthase and phosphorylase activities in rainbow trout hepatocytes. J Comp Physiol 165B:62–70. https://doi.org/10.1007/BF0026468
Carneiro NM, Amaral AD (1983) Effects of insulin and glucagon on plasma glucose levels and glycogen content in organs of the freshwater teleost Pimelodus maculatus. Gen Comp Endocr 49(1):115–121. https://doi.org/10.1016/0016-6480(83)90014-X
Wang ZQ, Zhang XH, Cefalu WT (2000) Chromium picolinate and biotin enhance glycogen synthesis and glycogen synthase gene expression in human skeletal muscle culture. Diabetes Res Clin Pract 50:395–395. https://doi.org/10.1016/S0168-8227(00)81348-0
Stahlhut HS, Whisnant CS, Lloyd KE, Baird EJ, Legleiter LR, Hansen SL, Spears JW (2006) Effect of chromium supplementation and copper status on glucose and lipid metabolism in Angus and Simmental beef cows. Anim Feed Sci Technol 128(3-4):253–265. https://doi.org/10.1016/j.anifeedsci.2005.11.002
Xi G, Xu Z, Wu SH, Chen SJ (2000) Effect of chromium picolinate on growth performance, carcass characteristics, serum metabolites and metabolism of lipid in pigs. Asian-Aust J Anim Sci 14:258–262
Wong RHF, Sul HS (2000) Insulin signaling in fatty acid and fat synthesis: a transcriptional perspective. Curr Opin Pharmacol 10(6):684–691. https://doi.org/10.1016/j.coph.2010.08.004
Shimano H, Horton JD, Shimomura I, Hammer RE, Brown MS, Goldstein JL (1997) Isoform 1c of sterol regulatory element binding protein is less active than isoform 1a in livers of transgenic mice and in cultured cells. J Clin Invest 99(3):846–854. https://doi.org/10.1111/acel.12576
Sul HS, Wang D (1998) Nutritional and hormonal regulation of enzymes in fat synthesis: studies of fatty acid synthase and mitochondrial glycerol-3-phosphate acyltransferase gene transcription. Annu Rev Nutr 18(1):331–351. https://doi.org/10.1146/annurev.nutr.18.1.331
Kim KH, Shin HJ, Kim K, Choi HM, Rhee SH (2007) Hepatitis B virus X protein induces hepatic steatosis via transcriptional activation of SREBP1 and PPARgamma. Gastroenterology 132(5):1955–1967. https://doi.org/10.1053/j.gastro.2007.03.039
Lushchak OV, Kubrak OI, Lozinsky OV, Storey JM, Storey KB, Lushchak VI (2009) Chromium (III) induces oxidative stress in goldfish liver and kidney. Aquat Toxicol 93(1):45–52. https://doi.org/10.1016/j.aquatox.2009.03.007
Landman GWD, Bilo HJD, Houweling ST, Kleefstra N (2014) Chromium does not belong in the diabetes treatment arsenal: current evidence and future perspectives. World J Diabetes 5(2):160–164. https://doi.org/10.4239/wjd.v5.i2.160
Komorowski JR, Greenberg D, Juturu V (2008) Chromium picolinate does not produce chromosome damage. Toxicol in Vitro 22(3):819–826. https://doi.org/10.1016/j.tiv.2007.12.007
Acknowledgements
The funding of the current research was supported by the National Science Foundation of China (31772820), the National Science Foundation of Jiangsu Province (BK20161143), and the Modern Agriculture Industrial Technology System special project-the national technology system for Conventional Freshwater Fish Industries (CARS-46).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The present study was approved by the Animal Care and Use Committee of Nanjing Agricultural University (Nanjing, China).
Rights and permissions
About this article
Cite this article
Ren, M., Mokrani, A., Liang, H. et al. Dietary Chromium Picolinate Supplementation Affects Growth, Whole-Body Composition, and Gene Expression Related to Glucose Metabolism and Lipogenesis in Juvenile Blunt Snout Bream, Megalobrama amblycephala. Biol Trace Elem Res 185, 205–215 (2018). https://doi.org/10.1007/s12011-018-1242-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-018-1242-0