Abstract
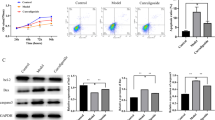
Increased intracellular free calcium ion (Ca2+) concentration induces excessive oxidative stress and apoptosis. Medical procedures such as zoledronic acid (Zol), bevacizumab (Bev), and dexamethasone (Dex) are usually used in the treatment of bone diseases (osteoporosis, Paget’s disease, etc.) and to prevent metastasis in the bone although the procedures induce osteonecrosis of the jaw through excessive production of reactive oxygen species (ROS). Recently, we observed regulator roles of selenium (Se) on apoptosis and Ca2+ entry through transient receptor potential vanilloid 1 (TRPV1) channels in the cancer cell lines. Therefore, Se may modulate Zol, Bev, and Dex-induced oxidative stress and apoptosis through regulation of TRPV1 channel. In the current study, we investigated the protective effects of Se on apoptosis and oxidative stress through TRPV1 in Zol, Bev, and Dex-induced osteoblast-like cell line. We used human osteoblast-like cell line (Saos-2), and the cells were divided into 12 groups as control, Zol, Bev, Dex, Se, Zol+Se, Bev+Se, Dex+Se, Zol+Dex, Zol+Dex+Se, Zol+Bev, and Zol+Bev+Se which were incubated with drugs (Zol, Bev, Dex, and Se) for 24 h. The cytosolic free Ca2+ concentration was increased by Zol, Bev, Dex, Zol+Bev, and Zol+Dex, although it was reduced by Se treatment. However, Zol, Bev, and Dex-induced increase in apoptosis, caspase 3, caspase 9, poly (ADP-ribose) polymerase 1 expression levels, and intracellular ROS production values in the cells were decreased by Se treatments. In conclusion, we observed that Zol, Bev, and Dex-induced apoptosis, mitochondrial oxidative stress, and calcium signaling are decreased in human osteoblast-like cell line by the Se treatment. Our findings may be relevant to the etiology and treatment of Zol, Bev, and Dex-induced osteonecrosis by Se.







Similar content being viewed by others
References
Kumar VS, Gopalakrishnan A, Naziroğlu M, Rajanikant GK (2014) Calcium ion—the key player in cerebral ischemia. Curr Med Chem 21:2065–2075
Berridge MJ, Bootman MD, Roderick HL (2003) Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 4(7):517–529
Kim B, Song YS (2016) Mitochondrial dynamics altered by oxidative stress in cancer. Free Radic Res 50:1065–1070
Sakallı Çetin E, Nazıroğlu M, Çiğ B, Övey İS, Aslan Koşar P (2017) Selenium potentiates the anticancer effect of cisplatin against oxidative stress and calcium ion signaling-induced intracellular toxicity in MCF-7 breast cancer cells: involvement of the TRPV1 channel. J Recept Signal Transduct Res 37:84–93
Pecze L, Blum W, Henzi T, Schwaller B (2016) Endogenous TRPV1 stimulation leads to the activation of the inositol phospholipid pathway necessary for sustained Ca(2+) oscillations. Biochim Biophys Acta 1863:2905–2915
Kozai D, Ogawa N, Mori Y (2014) Redox regulation of transient receptor potential channels. Antioxid Redox Signal 21(6):971–986
O'Neil RG, Brown RC (2003) The vanilloid receptor family of calcium permeable channels: molecular integrator of microenvironnemental stimuli. News Physiol Sci 18:226–231
Rousseau E, Cloutier M, Morin C, Proteau S (2005) Capsazepine, a vanilloid antagonist, abolishes tonic responses induced by 20-HETE on guinea pig airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 288(3):460–470
Koşar PA, Nazıroğlu M, Övey İS, Çiğ B (2016) Synergic effects of doxorubicin and melatonin on apoptosis and mitochondrial oxidative stress in MCF-7 breast cancer cells: involvement of TRPV1 channels. J Membr Biol 249:129–140
Pan L, Song K, Hu F, Sun W, Lee I (2013) Nitric oxide induces apoptosis associated with TRPV1 channel-mediated Ca(2+) entry via S-nitrosylation in osteoblasts. Eur J Pharmacol 715(1–3):280–285
Rossi F, Bellini G, Tortora C, Bernardo ME, Luongo L, Conforti A, Starc N, Manzo I, Nobili B, Locatelli F, Maione S (2015) CB(2) and TRPV(1) receptors oppositely modulate in vitro human osteoblast activity. Pharmacol Res 99:194–220
Little R, Muimo R, Robson L, Harris K, Grabowski PS (2011) The transient receptor potential ion channel TRPV6 is expressed at low levels in osteoblasts and has little role in osteoblast calcium uptake. PLoS One 6:e28166
Marx RE (2003) Pamidronate (Aredia) and Zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg 61:1115–1117
Ruggiero SL, Dodson TB, Mehrotra B (2014) American Association of Oral and Maxillofacial Surgeons on medication-related osteonecrosis of the jaw—2014 update. J Oral Maxillofac Surg 72(10):1938–1956
Koçer G, Nazıroğlu M, Çelik Ö, Önal L, Özçelik D, Koçer M, Sönmez TT (2013) Basic fibroblast growth factor attenuates bisphosphonate-induced oxidative injury but decreases zinc and copper levels in oral epithelium of rat. Biol Trace Elem Res 153:251–256
Senturk MF, Cimen E, Tuzuner Oncul AM, Cambazoglu M (2016) Oncologists awareness about bisphosphonate related osteonecrosis of the jaws. J Pak Med Assoc 66(7):880–883
Bagan J, Sáez GT, Tormos MC, Gavalda-Esteve C, Bagan L, Leopoldo-Rodado M, Calvo J, Camps C (2014) Oxidative stress in bisphosphonate-related osteonecrosis of the jaws. J Oral Pathol Med 43:371–377
Arai N, Inoue S, Tomihara K, Tsuno H, Noguchi M (2013) In vitro synergistic effects of zoledronic acid and calcium on viability of human epithelial cells. Oral Dis 19:200–205
Fardet L, Kassar A, Cabane J, Flahault A (2007) Corticosteroid-induced adverse events in adults: frequency, screening and prevention. Drug Saf 30(10):861–881
Chiu CT, Chiang WF, Chuang CY, Chang SW (2010) Resolution of oral bisphosphonates and steroid-related osteonecrosis of the jaw—a serial case analysis. J Oral Maxillofac Surg 68:1055–1063
Magremanne M, Reychler H (2014) Pentoxifylline and tocopherol in the treatment of yearly zoledronic acid-related osteonecrosis of the jaw in a corticosteroid-induced osteoporosis. J Oral Maxillofac Surg 72:334–337
Arbogast S, Ferreiro A (2010 Apr 1) Selenoproteins and protection against oxidative stress: selenoprotein N as a novel player at the crossroads of redox signaling and calcium homeostasis. Antioxid Redox Signal 12(7):893–904
Dai J, Liu H, Zhou J, Huang K (2016) Selenoprotein R protects human lens epithelial cells against D-galactose-induced apoptosis by regulating oxidative stress and endoplasmic reticulum stress. Int J Mol Sci 17:231
Çiğ B, Nazıroğlu M (2015) Investigation of the effects of distance from sources on apoptosis, oxidative stress and cytosolic calcium accumulation via TRPV1 channels induced by mobile phones and Wi-Fi in breast cancer cells. Biochim Biophys Acta 1848:2756–2765
Grynkiewicz C, Poenie M, Tsien RY (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260:3440–3450
Uğuz AC, Çig B, Espino J et al (2012) Melatonin potentiates chemotherapy-induced cytotoxicity and apoptosis in rat pancreatic tumor cells. J Pineal Res 53:91–98
Li P, Nijhawan D, Budihardjo I et al (1997) Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91:479–489
Gonzales CB, Kirma NB, De La Chapa JJ et al (2014) Vanilloids induce oral cancer apoptosis independent of TRPV1. Oral Oncol 50:437–447
Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL (2004) Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg 62:527–534
Street J, Lenehan B (2009) Vascular endothelial growth factor regulates osteoblast survival—evidence for an autocrine feedback mechanism. J Orthop Surg Res 4:19
Pakosch D, Papadimas D, Munding J, Kawa D, Kriwalsky MS (2013) Osteonecrosis of the mandible due to anti-angiogenic agent, bevacizumab. Oral Maxillofac Surg 17:303–306
Al Hadi H, Smerdon GR, Fox SW (2015) Hyperbaric oxygen therapy accelerates osteoblast differentiation and promotes bone formation. J Dent 43(3):382–388
Ren G, Ali T, Chen W, Han D, Zhang L, Gu X, Zhang S, Ding L, Fanning S, Han B (2016) The role of selenium in insulin-like growth factor I receptor (IGF-IR) expression and regulation of apoptosis in mouse osteoblasts. Chemosphere 144:2158–2164
Rodríguez-Lozano FJ, García-Bernal D, Ros-Roca Mde L, Algueró Mdel C, Oñate-Sánchez RE, Camacho-Alonso F, Moraleda JM (2015) Cytoprotective effects of melatonin on zoledronic acid-treated human mesenchymal stem cells in vitro. J Craniomaxillofac Surg 43(6):855–862
Tian L, Dang XQ, Wang CS, Yang P, Zhang C, Wang KZ (2013) Effects of sodium ferulate on preventing steroid-induced femoral head osteonecrosis in rabbits. J Zhejiang Univ Sci B 14(5):426–437
Guarneri V, Miles D, Robert N et al (2010) Bevacizumab and osteonecrosis of the jaw:incidence and association with bisphosphonate therapy in three large prospective trials in advanced breast cancer. Breast Cancer Res Treat 122:181
Vescovi P, Merigo E, Meleti M, Manfredi M, Fornaini C, Nammour S et al (2014) Conservative surgical management of stage I bisphosphonate-related osteonecrosis of the jaw. Int J Dent 107690:2014
Pan B, To LB, Farrugia AN, Findlay DM, Green J, Gronthos S et al (2004) The nitrogen-containing bisphosphonate, zoledronic acid, increases mineralization of human bone-derived cells in vitro. Bone 34:112–123
Moreau F, Guillet C, Massin P, Chevalier S, Gascan H, Basle MF (2007) Comparative effects of five bisphosphonates on apoptosis of macrophage cells in vitro. Biochem Pharmacol 73(5):718–723
Lang M, Zhou Z, Shi L, Niu J, Xu S, Lin W, Chen Z, Wang Y (2016) Influence of zoledronic acid on proliferation, migration, and apoptosis of vascular endothelial cells. Br J Oral Maxillofac Surg 54(8):889–893
Sharma RK, Chalam KV (2009) In vitro evaluation of bevacizumab toxicity on a retinal ganglion cell line. Acta Ophthalmol 87(6):618–622
Hein M, Graver S (2013) Tumor cell response to bevacizumab single agent therapy in vitro. Cancer Cell Int 13(1):94
Simon T, Coquerel B, Petit A, Kassim Y, Demange E, Le Cerf D, Perrot V, Vannier JP (2014) Direct effect of bevacizumab on glioblastoma cell lines in vitro. NeuroMolecular Med 16(4):752–771
Coelho MJ, Fernandes MH (2000) Human bone cell cultures in biocompatibility testing. Part II: effect of ascorbic acid, beta-glycerophosphate and dexamethasone on osteoblastic differentiation. Biomaterials 21(11):1095–1102
Guo S, Mao L, Ji F, Wang S, Xie Y, Fei H, Wang XD (2016) Activating AMP-activated protein kinase by an α1 selective activator compound 13 attenuates dexamethasone-induced osteoblast cell death. Biochem Biophys Res Commun 471(4):545–552
Ma YG, Liu WC, Dong S, Du C, Wang XJ, Li JS, Xie XP, Wu L, Ma DC, ZB Y, Xie MJ (2012) Activation of BK(Ca) channels in zoledronic acid-induced apoptosis of MDA-MB-231 breast cancer cells. PLoS One 7(5):e37451
Huang X, Huang S, Guo F, Xu F, Cheng P, Ye Y, Dong Y, Xiang W, Chen A (2016) Dose-dependent inhibitory effects of zoledronic acid on osteoblast viability and function in vitro. Mol Med Rep 13(1):613–622
Zafar S, Coates DE, Cullinan MP, Drummond BK, Milne T, Seymour GJ (2016) Effects of zoledronic acid and geranylgeraniol on the cellular behaviour and gene expression of primary human alveolar osteoblasts. Clin Oral Investig 20(8):2023–2035
Bayram H, Kenar H, Taşar F, Hasırcı V (2013) Effect of low level laser therapy and zoledronate on the viability and ALP activity of Saos-2 cells. Int J Oral Maxillofac Surg 42(1):140–146
Acknowledgments
The abstract of the study as poster presentation was published in the ACBID 10th International Congress, held 11 and 15 May 2016 in Antalya, Turkey (www.acbid.org). The study was supported by Scientific Project Unit of SDU (Protocol No: 4475-ÖYP-D2-15 and Protocol No: ÖYP05259-DR-12). All authors approved the final manuscript.
Author information
Authors and Affiliations
Contributions
MN and TY formulated the present hypothesis and were responsible for writing the report. TY was responsible for cell culture experiments. SÖ and AÖ were responsible for cytosolic Ca2+ release analyses. GK was made critical revision for the manuscript. The authors wish to thank the authors thank Muhammet Şahin, (Neuroscience Research Center, SDU, Isparta, Turkey) for helping with plate reader analyses.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Yazıcı, T., Koçer, G., Nazıroğlu, M. et al. Zoledronic Acid, Bevacizumab and Dexamethasone-Induced Apoptosis, Mitochondrial Oxidative Stress, and Calcium Signaling Are Decreased in Human Osteoblast-Like Cell Line by Selenium Treatment. Biol Trace Elem Res 184, 358–368 (2018). https://doi.org/10.1007/s12011-017-1187-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-017-1187-8