Abstract
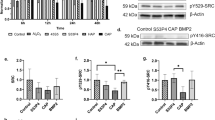
Magnesium has been investigated as a biodegradable metallic material. Increased concentrations of Mg2+ around magnesium implants due to biodegradation contribute to its satisfactory osteogenic capacity. However, the mechanisms underlying this process remain elusive. We propose that activation of the PI3K/Akt signalling pathway plays a role in the Mg2+-enhanced biological behaviours of osteoblasts. To test this hypothesis, 6, 10 and 18 mM Mg2+ was used to evaluate the stimulatory effect of Mg2+ on osteogenesis, which was assessed by evaluating cell adhesion, cell viability, ALP activity, extracellular matrix mineralisation and RT-PCR. The expression of p-Akt was also determined by western blotting. The results showed that 6 and 10 mM Mg2+ elicited the highest stimulatory effect on cell adhesion, cell viability and osteogenic differentiation as evidenced by cytoskeletal staining, MTT assay results, ALP activity, extracellular matrix mineralisation and expression of osteogenic differentiation-related genes. In contrast, 18 mM Mg2+ had an inhibitory effect on the behaviour of osteoblasts. Furthermore, 10 mM Mg2+ significantly increased the phosphorylation of Akt in osteoblasts. Notably, the aforementioned beneficial effects produced by 10 mM Mg2+ were abolished by blocking the PI3K/Akt signalling pathway through the addition of wortmannin. In conclusion, these results demonstrate that 6 mM and 10 mM Mg2+ can enhance the behaviour of osteoblasts, which is at least partially attributed to activation of the PI3K/Akt signalling pathway. Furthermore, a high concentration (18 mM Mg2+) showed an inhibitory effect on the biological behaviour of osteoblasts. These findings advance the understanding of cellular responses to biodegradable metallic materials and may attract greater clinical interest in magnesium.






Similar content being viewed by others
References
Niinomi M (2008) Metallic biomaterials. J Artif Organs 11:105–110
Niinomi M, Nakai M, Hieda J (2012) Development of new metallic alloys for biomedical applications. Acta Biomater 8:3888–3903
Staiger MP, Pietak AM, Huadmai J, Dias G (2006) Magnesium and its alloys as orthopedic biomaterials: a review. Biomaterials 27:1728–1734
Spitalny AD (2006) Bioabsorbable implants. Clin Podiatr Med Surg 23:673–694 v
Cui W, Beniash E, Gawalt E, Xu Z, Sfeir C (2013) Biomimetic coating of magnesium alloy for enhanced corrosion resistance and calcium phosphate deposition. Acta Biomater 9:8650–8659
Dorozhkin SV (2014) Calcium orthophosphate coatings on magnesium and its biodegradable alloys. Acta Biomater 10:2919–2934
Shadanbaz S, Dias GJ (2012) Calcium phosphate coatings on magnesium alloys for biomedical applications: a review. Acta Biomater 8:20–30
Witte F (2010) The history of biodegradable magnesium implants: a review. Acta Biomater 6:1680–1692
Touyz RM (2004) Magnesium in clinical medicine. Front Biosci 9:1278–1293
Witte F, Kaese V, Haferkamp H, Switzer E, Meyer-Lindenberg A et al (2005) In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 26:3557–3563
Kim BS, Kim JS, Park YM, Choi BY, Lee J (2013) Mg ion implantation on SLA-treated titanium surface and its effects on the behavior of mesenchymal stem cell. Mater Sci Eng C Mater Biol Appl 33:1554–1560
Wang G, Li J, Zhang W, Xu L, Pan H et al (2014) Magnesium ion implantation on a micro/nanostructured titanium surface promotes its bioactivity and osteogenic differentiation function. Int J Nanomedicine 9:2387–2398
Aziz SA, Davies M, Pick E, Zito C, Jilaveanu L et al (2009) Phosphatidylinositol-3-kinase as a therapeutic target in melanoma. Clin Cancer Res 15:3029–3036
Cantrell DA (2001) Phosphoinositide 3-kinase signalling pathways. J Cell Sci 114:1439–1445
Gu YX, Du J, Si MS, Mo JJ, Qiao SC et al (2013) The roles of PI3K/Akt signaling pathway in regulating MC3T3-E1 preosteoblast proliferation and differentiation on SLA and SLActive titanium surfaces. J Biomed Mater Res A 101:748–754
Guntur AR, Rosen CJ (2011) The skeleton: a multi-functional complex organ: new insights into osteoblasts and their role in bone formation: the central role of PI3Kinase. J Endocrinol 211:123–130
Li L, Xia Y, Wang Z, Cao X, Da Z et al (2011) Suppression of the PI3K-Akt pathway is involved in the decreased adhesion and migration of bone marrow-derived mesenchymal stem cells from non-obese diabetic mice. Cell Biol Int 35:961–966
Peng XD, Xu PZ, Chen ML, Hahn-Windgassen A, Skeen J et al (2003) Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev 17:1352–1365
Osyczka AM, Leboy PS (2005) Bone morphogenetic protein regulation of early osteoblast genes in human marrow stromal cells is mediated by extracellular signal-regulated kinase and phosphatidylinositol 3-kinase signaling. Endocrinology 146:3428–3437
Liu X, Bruxvoort KJ, Zylstra CR, Liu J, Cichowski R et al (2007) Lifelong accumulation of bone in mice lacking Pten in osteoblasts. Proc Natl Acad Sci U S A 104:2259–2264
Ghosh-Choudhury N, Abboud SL, Nishimura R, Celeste A, Mahimainathan L et al (2002) Requirement of BMP-2-induced phosphatidylinositol 3-kinase and Akt serine/threonine kinase in osteoblast differentiation and Smad-dependent BMP-2 gene transcription. J Biol Chem 277:33361–33368
Fujita T, Azuma Y, Fukuyama R, Hattori Y, Yoshida C et al (2004) Runx2 induces osteoblast and chondrocyte differentiation and enhances their migration by coupling with PI3K-Akt signaling. J Cell Biol 166:85–95
McGonnell IM, Grigoriadis AE, Lam EW, Price JS, Sunters A (2012) A specific role for phosphoinositide 3-kinase and AKT in osteoblasts? Front Endocrinol (Lausanne) 3:88
Su NY, Peng TC, Tsai PS, Huang CJ (2013) Phosphoinositide 3-kinase/Akt pathway is involved in mediating the anti-inflammation effects of magnesium sulfate. J Surg Res 185:726–732
Chen SD, Chen YB, Peng Y, Xu J, Chen SS et al (2010) Role of PI3K/Akt signaling in the protective effect of magnesium sulfate against ischemia-perfusion injury of small intestine in rats. Chin Med J 123:1447–1452
Feyerabend F, Witte F, Kammal M, Willumeit R (2006) Unphysiologically high magnesium concentrations support chondrocyte proliferation and redifferentiation. Tissue Eng 12:3545–3556
Yoshizawa S, Brown A, Barchowsky A, Sfeir C (2014) Magnesium ion stimulation of bone marrow stromal cells enhances osteogenic activity, simulating the effect of magnesium alloy degradation. Acta Biomater 10:2834–2842
Declercq H, Van den Vreken N, De Maeyer E, Verbeeck R, Schacht E et al (2004) Isolation, proliferation and differentiation of osteoblastic cells to study cell/biomaterial interactions: comparison of different isolation techniques and source. Biomaterials 25:757–768
Wen Y, Gu W, Cui J, Yu M, Zhang Y et al (2014) Platelet-rich plasma enhanced umbilical cord mesenchymal stem cells-based bone tissue regeneration. Arch Oral Biol 59:1146–1154
Tang CH, Yang RS, Huang TH, Lu DY, Chuang WJ et al (2006) Ultrasound stimulates cyclooxygenase-2 expression and increases bone formation through integrin, focal adhesion kinase, phosphatidylinositol 3-kinase, and Akt pathway in osteoblasts. Mol Pharmacol 69:2047–2057
Zhang Y, Zeng X, Zhang L, Zheng X (2007) Stimulatory effect of puerarin on bone formation through activation of PI3K/Akt pathway in rat calvaria osteoblasts. Planta Med 73:341–347
Wang J, Tang J, Zhang P, Li Y, Lai Y et al (2012) Surface modification of magnesium alloys developed for bioabsorbable orthopedic implants: a general review. J Biomed Mater Res B Appl Biomater 100:1691–1701
Chen Z, Mao X, Tan L, Friis T, Wu C et al (2014) Osteoimmunomodulatory properties of magnesium scaffolds coated with beta-tricalcium phosphate. Biomaterials 35:8553–8565
Zreiqat H, Howlett CR, Zannettino A, Evans P, Schulze-Tanzil G et al (2002) Mechanisms of magnesium-stimulated adhesion of osteoblastic cells to commonly used orthopaedic implants. J Biomed Mater Res 62:175–184
Wolf FI, Cittadini A (1999) Magnesium in cell proliferation and differentiation. Front Biosci 4:D607–D617
Castiglioni S, Cazzaniga A, Albisetti W, Maier JA (2013) Magnesium and osteoporosis: current state of knowledge and future research directions. Nutrients 5:3022–3033
Leidi M, Dellera F, Mariotti M, Banfi G, Crapanzano C et al (2012) Nitric oxide mediates low magnesium inhibition of osteoblast-like cell proliferation. J Nutr Biochem 23:1224–1229
Cai YL, Zhang JJ, Zhang S, Venkatraman SS, Zeng XT et al (2010) Osteoblastic cell response on fluoridated hydroxyapatite coatings: the effect of magnesium incorporation. Biomed Mater 5:054114
Lu J, Wei J, Yan Y, Li H, Jia J et al (2011) Preparation and preliminary cytocompatibility of magnesium doped apatite cement with degradability for bone regeneration. J Mater Sci Mater Med 22:607–615
Golshani-Hebroni S (2016) Mg(++) requirement for MtHK binding, and Mg(++) stabilization of mitochondrial membranes via activation of MtHK & MtCK and promotion of mitochondrial permeability transition pore closure: a hypothesis on mechanisms underlying mg(++)'s antioxidant and cytoprotective effects. Gene 581:1–13
Gu H, Guo F, Zhou X, Gong L, Zhang Y et al (2011) The stimulation of osteogenic differentiation of human adipose-derived stem cells by ionic products from akermanite dissolution via activation of the ERK pathway. Biomaterials 32:7023–7033
Weng L, Webster TJ (2013) Nanostructured magnesium has fewer detrimental effects on osteoblast function. Int J Nanomedicine 8:1773–1781
Knedler A, Ham RG (1987) Optimized medium for clonal growth of human microvascular endothelial cells with minimal serum. In Vitro Cell Dev Biol 23:481–491
Hallab NJ, Vermes C, Messina C, Roebuck KA, Glant TT et al (2002) Concentration- and composition-dependent effects of metal ions on human MG-63 osteoblasts. J Biomed Mater Res 60:420–433
Owen TA, Aronow M, Shalhoub V, Barone LM, Wilming L et al (1990) Progressive development of the rat osteoblast phenotype in vitro: reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J Cell Physiol 143:420–430
He LY, Zhang XM, Liu B, Tian Y, Ma WH (2016) Effect of magnesium ion on human osteoblast activity Braz J Med Biol Res:49
Mukherjee A, Rotwein P (2009) Akt promotes BMP2-mediated osteoblast differentiation and bone development. J Cell Sci 122:716–726
Liu HB, Meng QH, Huang C, Wang JB, Liu XW (2015) Nephroprotective effects of Polydatin against ischemia/reperfusion injury: a role for the PI3K/Akt signal pathway. Oxidative Med Cell Longev 2015:362158
Serrano-Nascimento C, da Silva TS, Nicola JP, Nachbar RT, Masini-Repiso AM et al (2014) The acute inhibitory effect of iodide excess on sodium/iodide symporter expression and activity involves the PI3K/Akt signaling pathway. Endocrinology 155:1145–1156
Kui L, Weiwei Z, Ling L, Daikun H, Guoming Z et al (2009) Ghrelin inhibits apoptosis induced by high glucose and sodium palmitate in adult rat cardiomyocytes through the PI3K-Akt signaling pathway. Regul Pept 155:62–69
Guan XH, Fu QC, Shi D, Bu HL, Song ZP, et al. (2014) Activation of spinal chemokine receptor CXCR3 mediates bone cancer pain through an Akt-ERK crosstalk pathway in rats. Exp Neurol
Acknowledgements
This work was supported by grants from the Research Fund for the National Natural Science Foundation of China (No. 81371933) to Lin Wang, the Xijing Zhutui Project (XJZT13M08) to Ya-Fei Feng and the Research Fund for the National Natural Science Foundation of China (No. 31170913/C10 02) to Wei Lei.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Jian Wang, Xiang-Yu Ma and Ya-Fei Feng contributed equally to this work
Rights and permissions
About this article
Cite this article
Wang, J., Ma, XY., Feng, YF. et al. Magnesium Ions Promote the Biological Behaviour of Rat Calvarial Osteoblasts by Activating the PI3K/Akt Signalling Pathway. Biol Trace Elem Res 179, 284–293 (2017). https://doi.org/10.1007/s12011-017-0948-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-017-0948-8