Abstract
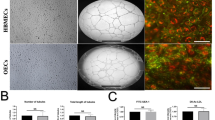
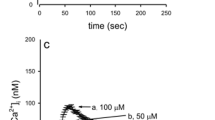
Many neurodegenerative diseases are related to copper although the effects on brain microvascular endothelial cells (BMECs) are poorly understood. In the present study, a primary BMEC culture model was established to evaluate the effects of copper on brain microvascular endothelial cells and whether claudin-1, claudin-3, claudin-5, and claudin-12 isoforms contribute to apoptosis and intrinsic antioxidant activity. Our results showed that copper ions had dual effects on BMECs by regulating intracellular reactive oxygen species (ROS) levels. Copper levels between 30 and 120 μM could enhance viability and promote proliferation. On the other hand, copper cytotoxicity was a result of apoptosis indicating a redox-independent manner of cell death. Expression levels of claudins were also regulated by copper in a concentration-dependent manner. We identified four claudin isoforms (1, 3, 5, and 12) and showed that their expression levels were regulated as a group by copper. Antioxidant activity of BMECs was also copper regulated, and superoxide dismutase and catalase were the main contributors to BMEC antioxidant functions. Together, our results indicated that copper had dual effects on BMEC growth and intrinsic antioxidant activities played a crucial role in BMEC survival and tight junction.





Similar content being viewed by others
References
Kaim W, Rall J (1996) Copper—a “modern” bioelement. Angew Chem 35:43–60
Maria C, Linder LW, Cerveza P, Cotton S, Shulze R, Lomeli N (1998) Copper transport. Am J Clin Nutr 67(suppl):965S–971S
Turski ML, Thiele DJ (2009) New roles for copper metabolism in cell proliferation, signaling, and disease. J Biol Chem 284(2):717–721. doi:10.1074/jbc.R800055200
de Romana DL, Olivares M, Uauy R, Araya M (2011) Risks and benefits of copper in light of new insights of copper homeostasis. J Trace Elem Med Biol Organ Soc Min Trace Elem 25(1):3–13. doi:10.1016/j.jtemb.2010.11.004
Tiffany-Castiglioni E, Hong S, Qian Y (2011) Copper handling by astrocytes: insights into neurodegenerative diseases. Int J Dev Neurosci Off J Int Soc Dev Neurosci 29(8):811–818. doi:10.1016/j.ijdevneu.2011.09.004
Scheiber IF, Dringen R (2013) Astrocyte functions in the copper homeostasis of the brain. Neurochem Int 62(5):556–565. doi:10.1016/j.neuint.2012.08.017
Svethlana Lutsenko AB, Hubbard AL (2010) Copper handling machinery of the brain. Metallomics Integrated Biometal Sci 2(9):596–608. doi:10.1039/c0mt00006j
Zatta P, Frank A (2007) Copper deficiency and neurological disorders in man and animals. Brain Res Rev 54(1):19–33. doi:10.1016/j.brainresrev.2006.10.001
Huidobro-Toro JP, Lorca RA, Coddou C (2008) Trace metals in the brain: allosteric modulators of ligand-gated receptor channels, the case of ATP-gated P2X receptors. Eur Biophys J EBJ 37(3):301–314. doi:10.1007/s00249-007-0230-7
Scheiber I, Dringen R, Mercer JFB (2013) Copper effects of deficiency and overload. In: Interrelations between essential metal ions and human diseases, vol 13. Springer, New York, pp. 360–381
Li F, Zhou X, Zhu J, Ma J, Huang X, Wong ST (2007) High content image analysis for human H4 neuroglioma cells exposed to CuO nanoparticles. BMC Biotechnol 7:66. doi:10.1186/1472-6750-7-66
Donnelly PS, Xiao Z, Wedd AG (2007) Copper and Alzheimer’s disease. Curr Opin Chem Biol 11(2):128–133. doi:10.1016/j.cbpa.2007.01.678
Scheiber IF, Mercer JF, Dringen R (2014) Metabolism and functions of copper in brain. Prog Neurobiol 116:33–57. doi:10.1016/j.pneurobio.2014.01.002
Bicker J, Alves G, Fortuna A, Falcao A (2014) Blood-brain barrier models and their relevance for a successful development of CNS drug delivery systems: a review. Eur J Pharm Biopharm Off J Arbeitsgemeinschaft Fur Pharmazeutische Verfahrenstechnik eV 87(3):409–432. doi:10.1016/j.ejpb.2014.03.012
Hartwig Wolburg AL (2002) Tight junctions of the blood–brain barrier development, composition and regulation. Vasc Pharmacol 38:323–337
Liebner S, Kniesel U, Kalbacher H, Wolburg H (2000) Correlation of tight junction morphology with the expression of tight junction proteins in blood-brain barrier endothelial cells. Eur J Cell Biol 79(10):707–717. doi:10.1078/0171-9335-00101
Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S (2003) Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol 161(3):653–660. doi:10.1083/jcb.200302070
Van Itallie CM, Anderson JM (2004) The molecular physiology of tight junction pores. Physiology 19:331–338. doi:10.1152/physiol.00027.2004
Chai Q, He WQ, Zhou M, Lu H, Fu ZF (2014) Enhancement of blood-brain barrier permeability and reduction of tight junction protein expression are modulated by chemokines/cytokines induced by rabies virus infection. J Virol 88(9):4698–4710. doi:10.1128/JVI.03149-13
Freese C, Reinhardt S, Hefner G, Unger RE, Kirkpatrick CJ, Endres K (2014) A novel blood-brain barrier co-culture system for drug targeting of Alzheimer’s disease: establishment by using acitretin as a model drug. PLoS One 9(3):e91003. doi:10.1371/journal.pone.0091003
Takeshita Y, Obermeier B, Cotleur A, Sano Y, Kanda T, Ransohoff RM (2014) An in vitro blood-brain barrier model combining shear stress and endothelial cell/astrocyte co-culture. J Neurosci Methods 232:165–172. doi:10.1016/j.jneumeth.2014.05.013
Wilhelm I, Krizbai IA (2014) In vitro models of the blood-brain barrier for the study of drug delivery to the brain. Mol Pharm 11(7):1949–1963. doi:10.1021/mp500046f
Burek M, Salvador E, Forster CY (2012) Generation of an immortalized murine brain microvascular endothelial cell line as an in vitro blood brain barrier model. J Visualized Exp JoVE 66:e4022. doi:10.3791/4022
Liu Y, Xue Q, Tang Q, Hou M, Qi H, Chen G, Chen W, Zhang J, Chen Y, Xu X (2013) A simple method for isolating and culturing the rat brain microvascular endothelial cells. Microvasc Res 90:199–205. doi:10.1016/j.mvr.2013.08.004
Mokgokong R, Wang S, Taylor CJ, Barrand MA, Hladky SB (2014) Ion transporters in brain endothelial cells that contribute to formation of brain interstitial fluid. Pflugers Arch - Eur J Physiol 466(5):887–901. doi:10.1007/s00424-013-1342-9
Prasad Chennazhy K, Krishnan LK (2005) Effect of passage number and matrix characteristics on differentiation of endothelial cells cultured for tissue engineering. Biomaterials 26(28):5658–5667. doi:10.1016/j.biomaterials.2005.02.024
Scheiber IF, Mercer JF, Dringen R (2010) Copper accumulation by cultured astrocytes. Neurochem Int 56(3):451–460. doi:10.1016/j.neuint.2009.12.002
Ferruzza S, Scacchi M, Scarino ML, Sambuy Y (2002) Iron and copper alter tight junction permeability in human intestinal Caco-2 cells by distinct mechanisms. Toxicol in Vitro Int J Publ Assoc BIBRA 16(4):399–404
Sasaki TKK, Saijo-Kurita K, Ohno T (1992) Detergent cytotoxicity: simplified assay of cytolysis by measuring LDH activity. Toxicol in Vitro 6(5):451–457
Shi XLZY, Jiao YD, Shi TR, Yang XB (2013) ROS-dependent mitochondria molecular mechanisms underlying antitumor activity of Pleurotus abalonus acidic polysaccharides in human breast cancer MCF-7 cells. PLoS One 8(5):e64266
Zhang C, Chi YL, Wang PY, Wang YQ, Zhang YX, Deng J, Lv CJ, Xie SY (2012) miR-511 and miR-1297 inhibit human lung adenocarcinoma cell proliferation by targeting oncogene TRIB2. PloS one 7(10):e46090. doi:10.1371/journal.pone.0046090
Zhang Y, Chen L, Li F, Wang H, Yao Y, Shu J, Ying MZ (2016) Cryptotanshinone protects against adriamycin-induced mitochondrial dysfunction in cardiomyocytes. Pharm Biol 54(2):237–242. doi:10.3109/13880209.2015.1029052
Qian LB, Wang HP, Qiu WL, Huang H, Bruce IC, Xia Q (2006) Interleukin-2 protects against endothelial dysfunction induced by high glucose levels in rats. Vasc Pharmacol 45(6):374–382. doi:10.1016/j.vph.2006.06.002
Liu L, Liu Y, Cui J, Liu H, Liu YB, Qiao WL, Sun H, Yan CD (2013) Oxidative stress induces gastric submucosal arteriolar dysfunction in the elderly. World J Gastroenterol WJG 19(48):9439–9446. doi:10.3748/wjg.v19.i48.9439
Luo Z, Wang B, Liu M, Jiang K, Liu M, Wang L (2014) Effect of dietary supplementation of vitamin C on growth, reactive oxygen species, and antioxidant enzyme activity of Apostichopus japonicus (Selenka) juveniles exposed to nitrite. Chin J Oceanol Limnol 32(4):749–763. doi:10.1007/s00343-014-3310-4
Hou H, Li B, Zhao X, Zhuang Y, Ren G, Yan M, Cai Y, Zhang X, Chen L (2009) The effect of pacific cod (Gadus macrocephalus) skin gelatin polypeptides on UV radiation-induced skin photoaging in ICR mice. Food Chem 115(3):945–950. doi:10.1016/j.foodchem.2009.01.015
Pardridge WM (2005) The blood-brain barrier: bottleneck in brain drug development. NeuroRx J Am Soc Exp NeuroTher 2(1):3–14. doi:10.1602/neurorx.2.1.3
Kaushik Banerjee SB, Satyajit Das, Abhinaba Sinha, Manas Kumar Biswas, Soumita Kumar Choudhuri (2016) Induction of intrinsic and extrinsic apoptosis through oxidative stress in drug resistant cancer by a newly synthesized Schiff base copper chelate. Free radical research:1–55. doi:10.3109/10715762.2015.1136062
Cherny RA, Barnham KJ, Lynch T, Volitakis I, Li QX, McLean CA, Multhaup G, Beyreuther K, Tanzi RE, Masters CL, Bush AI (2000) Chelation and intercalation: complementary properties in a compound for the treatment of Alzheimer’s disease. J Struct Biol 1(2–3):209–216. doi:10.1006/jsbi.2000.4285
Brady DC, Crowe MS, Turski ML, Hobbs GA, Yao X, Chaikuad A, Knapp S, Xiao K, Campbell SL, Thiele DJ, Counter CM (2014) Copper is required for oncogenic BRAF signalling and tumorigenesis. Nature 509(7501):492–496. doi:10.1038/nature13180
MacDonald G, Nalvarte I, Smirnova T, Vecchi M, Aceto N, Dolemeyer A, Frei A, Lienhard S, Wyckoff J, Hess D, Seebacher J, Keusch JJ, Gut H, Salaun D, Mazzarol G, Disalvatore D, Bentires-Alj M, Di Fiore PP, Badache A, Hynes NE (2014) Memo is a copper-dependent redox protein with an essential role in migration and metastasis. Sci Signal 7(329):ra56. doi:10.1126/scisignal.2004870
Finkel T (2011) Signal transduction by reactive oxygen species. J Cell Biol 194(1):7–15. doi:10.1083/jcb.201102095
Liochev SI (2013) Reactive oxygen species and the free radical theory of aging. Free Radic Biol Med 60:1–4. doi:10.1016/j.freeradbiomed.2013.02.011
Murphy MP, Holmgren A, Larsson NG, Halliwell B, Chang CJ, Kalyanaraman B, Rhee SG, Thornalley PJ, Partridge L, Gems D, Nystrom T, Belousov V, Schumacker PT, Winterbourn CC (2011) Unraveling the biological roles of reactive oxygen species. Cell Metab 13(4):361–366. doi:10.1016/j.cmet.2011.03.010
Derkus B, Emregul E, Emregul KC (2015) Copper–zinc alloy nanoparticle based enzyme-free superoxide radical sensing on a screen-printed electrode. Talanta 134:206–214. doi:10.1016/j.talanta.2014.11.003
Richard C, Jin JL (2010) Vascular nitric oxide formation and function. J Blood Med 1:147–162
Moncada S, Higgs EA (2006) The discovery of nitric oxide and its role in vascular biology. Br J Pharmacol 147(Suppl 1):S193–S201. doi:10.1038/sj.bjp.0706458
D’Autreaux B, Toledano MB (2007) ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol 8(10):813–824. doi:10.1038/nrm2256
Schieber M, Chandel NS (2014) ROS function in redox signaling and oxidative stress. Curr Biol CB 24(10):R453–R462. doi:10.1016/j.cub.2014.03.034
Wellen KE, Thompson CB (2010) Cellular metabolic stress: considering how cells respond to nutrient excess. Mol Cell 40(2):323–332. doi:10.1016/j.molcel.2010.10.004
Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M (2006) Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 160(1):1–40. doi:10.1016/j.cbi.2005.12.009
Liochev SI, Fridovich I (2002) The Haber-Weiss cycle—70 years later: an alternative view. Redox Report. Commun Free Radic Res 7(1):55–57 . doi:10.1179/135100002125000190author reply 59-60
Moriwaki H, Osborne MR, Phillips DH (2008) Effects of mixing metal ions on oxidative DNA damage mediated by a Fenton-type reduction. Toxicol in Vitro Int Journal Publ Assoc BIBRA 22(1):36–44. doi:10.1016/j.tiv.2007.07.011
Hawkins BT, Davis TP (2005) The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev 57(2):173–185. doi:10.1124/pr.57.2.4
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (Nos. 31572585, 30871900, 30671550).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Experimental procedures involving animals were performed with the approval of the Laboratory Animal Management and Use Committee and Ethics Committee of South China Agricultural University.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Wang, J., Chen, J., Tang, Z. et al. The Effects of Copper on Brain Microvascular Endothelial Cells and Claudin Via Apoptosis and Oxidative Stress. Biol Trace Elem Res 174, 132–141 (2016). https://doi.org/10.1007/s12011-016-0685-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-016-0685-4