Abstract
Copper (Cu) is required for basically all biochemical and physiological processes in the body. The aim was to evaluate the effects of different sources of dietary copper on jejunal epithelium histomorphometry in adolescent rats. Male rats at the age of 5 weeks were used in the 12-week experiment. The control group was fed with standard diet providing the required Cu level (5 mg/kg body weight (bw) per day) in an inorganic form (sulfate) covered 100 % of daily demand, and the other three groups were supplemented with Cu-glycine complex covered 50, 75, and 100 % daily demand. Basal hematological and plasma biochemical analyses as well as histomorphometric examinations of the jejunal epithelium and liver were performed. Cu given in the organic form in 100 % of daily demand depressed the muscular and submucosa layer and the crypt depth (P < 0.05) without an influence of the innervation of the jejunum. In turn, organic Cu given in 75 % of daily demand did not influence the intestinal morphology in adult rats. Dietary organic Cu given to rats covering the daily demand in 50 or 75 % appears to be less harmful with regard to the intestinal epithelium than when administered in 100 % of daily demand.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well known that trace minerals such as copper (Cu) are required for normal functioning of basically all biochemical and physiological processes in the body. It is one of the most abundant essential trace minerals after iron and zinc. The average content of Cu in tissues of growing mammals is three times higher than in adults [1, 2]. The required amount and tissue content of Cu in the body alters throughout the lifetime in animals and humans. The liver and kidney contain the highest amount of Cu among all tissues [1, 2]. Copper as a trace element is needed to maintain optimal body function, growth, reproduction, and immune response, determining in general the health status [3].
The imbalance of Cu homeostasis causes different health problems. The deficiency of Cu leads to anemia and bone malformation, problems with locomotor function, and increases the risk of developing osteoporosis in later life [3, 4]. Moreover, its deficit causes alterations in cholesterol and glucose metabolism with a resulting increase in the glucose and cholesterol level [3, 4]. On the other hand, Cu excess is toxic and generates free radicals [5]. Elevated Cu concentration can cause toxicosis and degeneration of liver tissue as well as brain damage like in Wilson’s disease [3, 6].
Cu absorption in the amount of 50 to 80 % of ingested Cu takes place in the small intestine [3]. Moreover, this absorption is influenced by iron, manganese, zinc, and cobalt [3]. Additionally, the chemical form in which the microelement is present changes its bioavailability [7–9]. Copper in animal and human diet can be given in inorganic sources (as copper sulfate or carbonate) or organic sources as amino acid chelate with higher Cu bioavailability due to the fact that additional connection with amino acid is not required at the brush border and the membrane transport is more rapid [3, 7, 10, 11].
Animal studies show that reduction of the concentrations of Cu typically supplemented to pig diets greatly decreases fecal mineral excretion without negatively affecting pig performance from weaning through development [12]. This is in agreement with a study where the use of lower levels of diet minerals caused a 21 % reduction in excretion of Cu [13]. It seems that farm animals are overfed trace minerals, and the use of a mineral chelate allows maintenance of performance, concomitantly reducing the mineral content in manure [13, 14]. The cause of the reduction in the level of trace minerals in the organic form could be related to the fact that rats administered with Cu chelate in 100 % of daily demand showed considerable damage to liver (large increase in apoptotic cells, ballooning degeneration, and vacuolization) compared to diets containing the same levels of Cu provided from sulfate [15]. Despite the fact that the role of Cu in animal and human health is well established, there is no knowledge about the influence of different sources thereof in the diet on the intestinal epithelium.
Dietary trace mineral sources and levels were administered to adolescent rats to evaluate their developmental responses during the growth phase on the basis of the histomorphometric analysis of the jejunal epithelium.
Materials and Methods
The experimental procedures used throughout this study were approved by the Local Ethics Committee on Animal Experimentation of University of Life Sciences of Lublin, Poland. The rats were maintained in an animal house according to the guidelines of this committee. Experiment complied with the Guiding Principles for Research Involving Animals.
Animal, Breeding, and Experimental Design
Male Wistar rats (n = 48) at the age of 5 weeks at the start of the experiment were used in the experiment lasted 12 weeks. Clinically healthy rats were individually kept in Macrolon cages (Bioscape, Emmendingen, Germany) at 21 ± 1 °C and 55 % humidity and 12-h light and dark cycles. All animals had free access to water and fed ad libitum. Adolescent rats were randomly divided into the control group (n = 12), in which Cu was provided by conventional inorganic salts (sulfate) and three experimental groups (each n = 12) fed different level of the organic Cu as Cu-glycine complex. The control group was fed with standard diet provided Cu in 100 % of daily demand from sulfate (5 mg/kg body weight (bw) per day; LSM, Agropol S.J., Motycz, Poland). The composition of basal diet is crude protein min. 14.5 %, crude fat min. 1.5 %, ash 10 %, and crude fiber min. 5 %. The content of vitamin and mineral premixes of the diet is presented in Table 1. On the other hand, experimental animals were fed with the same standard diet, but the experimental treatment contained 0 % of Cu in the diet. Copper was given to distilled water as Cu amino acid chelate (Cu-glycine complex) in the amount of 0.025 mg Cu/L H2O in the OG100 group (covered 100 % of daily demand), in the amount of 0.01875 mg Cu/L H2O in the OG75 group (covered 75 % of daily demand) and in the amount of 0.0125 mg Cu/L H2O in the OG50 group (covered 50 % of daily demand). The Cu-glycine complex covering 100 % of daily demand and the inorganic salts (sulfate) contained an equivalent mineral concentration. Water consumption during 24 h for the four groups of rats was measured before the beginning of the experiment, and the data were used to calculate the needed amount of Cu. These data, combined with body weight, Cu content in the chelate, and the amount of the chelate covering Cu demand, were used to calculate the amount of Cu at the dose of 5 mg/kg bw/day in distilled water. At the end of experiment, rats were fasted for 24 h and euthanized one by one with carbon dioxide inhalation and by dislocation of the spine.
Hematological and Plasma Biochemical Analyses
Blood samples were collected carefully for hematological and blood plasma biochemical analyses by the heart venipuncture. Hematological analyses were performed with the use of an automatic hematological analyzer MS9 (Melet Schloesing Laboratories, France). The numbers of white and red blood cells (WBC and RBC), hemoglobin concentration (Hb), and hematocrit (HT) were determined.
The plasma was immediately separated by centrifugation and stored at −25 °C for further analysis. The plasma concentration of Cu, Fe, and Zn was determined by the colorimetric method using a Metrolab 2300 GL unit (Metrolab SA, Buenos Aires, Argentina) and ready-made sets produced by the company BioMaxima (Lublin, Poland).
Total protein, glucose, total cholesterol (TC), triacylglicerol (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), lactate dehydrogenase (LDH), and alkaline phosphatase (ALP) were determined by the colorimetric method using a Metrolab 2300GL random access biochemical analyzer (Metrolab SA, Buenos Aires, Argentina) and tests by BioMaxima (Lublin, Poland).
Tissue Collection and Histomorphometrical Analysis
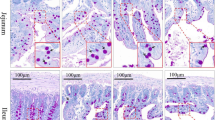
A 15-mm-long segment from the same point in 50 % of the total jejunum length was taken from each animal of each group and was subjected to histology. Briefly, these samples of small intestine were opened along the mesenteric border, placed flat without stretching in standard histopathological cassettes (Bio-Optica Milano S.p.A, Mediolan, Italy) and fixed in 4 % buffered formaldehyde (pH 7.0) for 24 h, then dehydrated in graded series of ethanol and embedded in paraffin. Twenty cross sections, 4 μm thick, were cut with a microtome (Microm HM 360, Microm, Walldorf, Germany) with 20 μm interval after each five slices and were placed on poly-L-lysine-coated slides (Menzel Glasser, Braunschweig, Germany) and then stained using Masson’s trichrome method to differentiate the small intestine wall layers [16]. Microscopic images were collected using a microscope (Axiovert 200 M, Carl Zeiss, Jena, Germany). Objective magnifications of 4×, 10×, 20×, and 40× were used to show the different intestinal structures and to collect images of the examined tissues from each specimen for further analysis. The structure of the small intestine wall was examined under microscopic observation and with the use of graphic analysis software Olympus cellSens Version 1.5 (Olympus, Tokyo, Japan).
The following morphometric variables in the intestine were analyzed: mucosa, submucosa, and myenteron (longitudinal and transversal lamina) thickness (30 measurements, with the use of straight line, from the bottom to the top of each layer, of every sample); villar epithelium thickness (with the use of straight line, from the bottom to the top of epithelium cells, from 30 villi of each animal); enterocyte number per 100 μm of the villus (from 30 villi of each animal); crypt depth (defined as the depth of the invagination between adjacent villi from the bottom of the crypt to the base of villi); crypt width (measured in the middle of the crypt depth); the number of crypts (active: showing mitoses and Paneth cells, having an open internal space and access to the intestinal lumen; inactive: showing no mitoses and Paneth cells, having a closed internal space; total: active plus inactive crypts); villar length (from the tip of the villi to the villous-crypt junction); villar thickness (measured in the middle of villar height); the number of villi (100 well-defined villi from each animal); and small intestine absorptive surface as described previously [17].
Statistical Analysis
All the results are expressed as means ± standard deviation (SD). Differences between the means were tested with the one-way ANOVA and post hoc Tukey’s test as the correction for multiple comparisons. Normal distribution of data was examined using the W. Shapiro-Wilk test, and equality of variance was tested by the Brown-Forsythe test. A P value of less than 0.05 was considered statistically significant. All statistical analyses were carried out by means of Statistica 12 software (StatSoft, Inc., Tulsa, OK, USA; http://www.statsoft.com).
Results
Food Consumption and Body Mass
Food consumption was measured daily in control animals and treated with Cu, and there was no differences (data not shown).
The average initial body mass of all the rats reached the value of 225 ± 30 g. The final body mass of the control rats and animals treated with the organic Cu source (regardless of the amount of daily demand) was similar and reached the value of 405.0 ± 50.0, 406.6 ± 39.0, 419.0 ± 50.0, and 411.9 ± 42.4 g in the control, OG100, OG75, and OG50 group, respectively.
The Content of Cu in Blood Plasma
The Cu plasma concentration of the control rats and animals supplemented with the organic form of Cu (irrespective of the amount of Cu in the diet) did not differ between the groups (Table 2).
Blood Hematology and Blood Plasma Biochemistry
The basal blood hematology in the control group did not differ from the value obtained in the groups treated with Cu-glycine complex in 100, 75, or 50 % of daily demand except that the number of white blood corpus was decreased in the OG75 group (Table 2). There was no difference in basal biochemical parameters between the control group and rats treated with the organic Cu (independently on the amount of daily demand), except glucose concentration, which significantly increased in the group fed with the organic form in 100 % of daily demand (Table 2).
Gastrointestinal Track Morphology
The intake of Cu in Cu-glycine complex at the level of 100 % of daily demand resulted in reduction of the thickness of the villar epithelium compared to the control group (supplemented with Cu in the inorganic form at the level of 100 % of daily demand) and the group supplemented with the Cu amino acid chelate in 75 and 50 % of daily demand (Table 3). The number of enterocytes also decreased in this group compared to the control group (supplemented with Cu in the inorganic form at the level of 100 % of daily demand) and the group supplemented with the Cu amino acid chelate in 75 % of daily demand (Table 3).
The Cu supplementation in the organic form did not influence the histomorphometric parameters of the nerve plexus in the jejunum compared to the Cu supplementation in the inorganic form (Table 4).
Discussion
Cu deficiency can occur early in life when elevated requirements are evident during rapid growth. For the first time, the full spectrum of Cu deficiency was shown in 1964 when Cu was considered the cause of anemia. Then, infants and children receiving copper-free total parenteral nutrition served to clearly define copper as an essential nutrient for human infants. Further, tissue Cu deficiency as a result of an inherited defect of Cu transport was also described as Menkes-Syndrom [18]. On the other hand, Cu accumulation in the liver is observed in another inherited disorder related to Cu metabolism, namely Wilson’s disease with a simultaneously decreased Cu serum concentration [3]. However, sometimes, dietary supplements containing Cu are the main sources of this trace element. Thus, it is necessary to examine the effects of the supplementation of Cu on intestine histomorphometric changes in conditions of full coverage of the daily requirement or low, deficient intake of Cu in relation to the daily requirement defined as providing a minimum of given trace element in order to cover a required amount to maintain normal homeostasis for a 24-h period in rats. In the current study, two of the diets supplemented with organic Cu as Cu-Gly covered the daily demand in 75 and 50 %.
The obtained results demonstrated that there was no direct effect of the dietary Cu-glycine complex in 100, 75, and 50 % of daily demand on feed intake in adolescent rats. Additionally, Cu supplementation at the experimentally lowered level in Cu-glycine complex did not affect the body weight. This is in agreement with an earlier study also performed on adolescent rats supplemented with Cu as chelate at the level of 25, 50, and 75 % of the required daily amount [15]. However, this result differed from others reporting that Cu deficiency in relation to daily requirement leads to a reduced body mass because Cu is essential for normal growth and development [18–20].
The current study indicated that supplementation with Cu did not influence biochemical and basal hematologicalal parameters in the adolescent rats (irrespective of the Cu amount in the diet). It is worth mentioning that Cu-deficient diet does not affect the final body mass of adult rats. Moreover, in our adolescent animals, there was no difference in the Cu plasma concentration between the control group supplemented with the Cu sulfate in 100 % daily demand and the groups supplemented with Cu-glycine complex at the level below the daily demand for the rat. This may indicate that there was sufficient Cu absorption from the intestine in adolescent rats. A similar study showed that in rats fed with Cu amino acid chelate lowered to 50 %, and even to 25 % of daily demand, there was no Cu liver deficiency [15]. Moreover, numerous studies in animals and human volunteers show a link between Cu deficiency and altered lipid metabolism [21–24]. An increased concentration of total cholesterol and LDL cholesterol and a reduction of HDL cholesterol were observed in subjects fed an experimental Cu-low diet because of more rapid synthesis and clearance thereof into blood plasma and a limited cholesterol pool for excretion as biliary steroids [25]. A low Cu intake was also shown to diminish glucose tolerance [18].
Our study performed on adolescent rats showed that Cu given in the diet in Cu-glycine complex irrespective of its dose did not alter glucose and lipid metabolism. The lack of differences in the glucose concentration between the investigated groups can indicate that our adolescent rats were not characterized by lowered activity of enzymes responsible for metabolic transformation of glycogen in the body and abnormal accumulation thereof, i.e., glycogenosis.
Copper homeostasis and status are regulated at the whole body level mainly by intestinal absorption. Copper supplementation in the broiler diet at a dose higher than 250 mg/kg depressed the height of villi and thickened the muscular layer in the duodenum [26]. Study performed on weanling pigs shows that duodenal villus height was reduced in the group supplemented with Cu sulfate, but inorganic salt was given in higher amount (225 mg of Cu/kg of a diet) than the minimum daily dose by American standards that recommended 6–125 ppm depending on production cycle [27, 28]. A different Cu study showed that rats fed with diet supplemented with CuSO4 at the dose of 80 mg/kg body weight had no effects on the villus height and crypt depth of small intestinal mucosa [29]. However, Cu-deficient diet (less than 1 mg Cu/kg) in cattle resulted in lesions of the small intestine [30]. The morphology of villi changes depending on the examined segment of the intestine and its function, age, and diet, and environmental factors. Rat studies showed that Cu deficiency affected the enteric nervous system without significant changes in secretory function [31].
Our study showed that the Cu-deficient diet in rats did not affect the enteric nervous system and probably did not influence its function (Table 4). Nevertheless, on the basis of the histomorphometric analysis of the jejunum, the current study indicated that Cu given in the organic form covering 100 % of daily demand in adolescent rats depressed the number and height of enterocytes (Table 3). In turn, organic Cu given in the amount lowered to 75 or 50 % of daily demand did not influence the morphology of enterocytes. Changes in the morphology of enterocytes, however, can have a decisive influence on their function related to contact digestion within the brush-border membrane and absorption of the end products of digestion. Moreover, a tendency to a decreased number and height of villi was observed, especially in the groups supplemented with the organic form of Cu (in the amount of 100 and 75 % of daily demand). Even slight changes in the shape or length of the villi substantially affect the absorptive area. It should be noted that the growth of a young villus is an effect of decreased exfoliation of apoptotic cells at the top of the villi but not increased mitotic activity in the zone of renewal in the intestinal crypts. Probably, when enterocytes move from the renewal zone toward the top of the villus, epithelial cells mature, differentiate rapidly, and undergo apoptosis leading to villus shortening when organic Cu is present in the diet in the amount of 100 or 75 % of daily demand. Despite the increased proliferation and migration of enterocytes, the renewal of damaged and massively exfoliated epithelium is insufficient and might lead to flattening and villar atrophy. Our rats fed with organic Cu showed a tendency to reduction of the number of villi. On the other hand, this might be caused by the decline in the renewal process as an effect of the decrease in the number of active crypts and the increase in the number of inactive crypt in rats fed with diet containing organic Cu in the amount of 100 % of daily demand.
Conclusions
Cu given to adolescent rats in the diet in the organic form covering an amount of the full daily demand in 100 % appears to be more harmful with regard to the intestinal epithelium than when administered in 75 or 50 % of daily demand and compared to the inorganic dietary Cu supplementation in the amount of 100 % of daily demand. Organic dietary Cu supplementation probably influenced the villar developmental process and led to massive cell exfoliation on the upper half of the villus tip, thereby shortening villar length and the number of enterocytes. The damage of jejuna epithelium can cause the disease and infection in the intestinal tract, the development of pathogenic intestinal microflora, and decreased immunity, which can lead to the development of food allergies. From the point of view of the digestive function of the intestinal epithelium, it is beneficial to reduce the daily dose of organic Cu as a dietary supplement.
References
Peňa MMO, Lee J, Thiele DJ (1999) A delicate balance: homeostatic control of copper uptake and distribution. J Nutr 1129:1251–1260
Rinaldi AC (2000) Meeting report—copper research at the top. Biometals 13:9–13
Linder MC, Hazegh-Azam M (1996) Copper biochemistry and molecular biology. Am J Clin Nutr 63:797S–811S
Fields M, Ferretti RJ, Reiser S, Smith JC Jr (1984) The severity of copper deficiency in rats is determined by the type of dietary carbohydrate. Exp Biol Med 175:530–537
Brewer GJ (2010) Copper toxicity in the general population. Clin Neurophysiol 121:459–460. doi:10.1016/j.clinph.2009.12.015
Roberts EA, Schilsky ML (2008) Diagnosis and treatment of Wilson disease: an update. Hepatol 47:2089–2111. doi:10.1002/hep.22261
Männer K, Simon O, Schlegel P (2006) Effects of different iron, manganese, zinc and copper sources (sulfates, chelates, glycinates) on their bioavailability in early weaned piglets. In: Rodehutscord M (ed) Tagung Schweine - und Geflügelernährung, 9th edn. Universität Halle-Wittenberg, Germany
Świątkiewicz S, Koreleski J, Hong DQ (2001) The bioavailability of zinc from inorganic and organic sources in broiler chickens as affected by addition of phytase. J Anim Feed Sci 10:317–328
Andersen O (2004) Chemical and biological considerations in the treatment of metal intoxications by chelating agents. Mini Rev Med Chem 4:1–21. doi:10.2174/1389557043487583
Veum TL, Carlson MS, Wu CW, Bollinger DW, Ellersieck MR (2004) Trace minerals proteinate in weanling pig diets for enhancing growth performance and reducing fecal copper excretion compared with copper sulphate. J Anim Sci 82:1062–1070
Bengtsson H, Öborn I, Jonsson S, Nilsson I, Andersson A (2003) Field balances of some mineral nutrients and trace elements in organic and conventional dairy farming—a case study at Öjebyn, Sweden. Eur J Agron 20:101–116. doi:10.1016/S1161-0301(03)00079-0
Creech BL, Spears JW, Flowers WL, Hill GM, Lloyd KE, Armstrong T, Engle TE (2004) Effect of dietary trace mineral concentration and source (inorganic vs. chelated) on performance, mineral status, and fecal mineral excretion in pigs from weaning through finishing. J Anim Sci 82:2140–2147, http://jas.fass.org/cgi/content/full/82/7/2140
Leeson S, Caston L (2008) Using minimal supplements of trace minerals as a method of reducing trace mineral content of poultry manure. Anim Feed Sci Technol 142:339–347. doi:10.1016/j.anifeedsci.2007.08.004
Jondreville C, Revy PS, Dourmad JY (2003) Dietary means to better control the environmental impact of copper and zinc by pigs from weaning to slaughter. Livest Prod Sci 84:147–156. doi:10.1016/j.livprodsci.2003.09.011
Tomaszewska E, Dobrowolski P, Kwiecień M, Burmańczuk N, Badzian B, Szymańczyk S, Kurlak P (2014) Alterations of liver histomorphology in relation to copper supplementation in inorganic and organic form in growing rats. Bull Vet Inst Pulawy 58:479–486. doi:10.2478/bvip-2014-0073
Suvara SK, Layton C, Bancroft JD (2013) Bancroft's theory and practice of histological techniques 7th edition. Churchill Livingstone Elsevier
Kisielinski K, Willis S, Prescher A, Klosterhalfen B, Schumpelick V (2002) A simple new method to calculate small intestine absorptive surface in the rat. Clin Exp Med 2:131–135. doi:10.1007/s102380200018
Uauy R, Olivares M, Gonzalez M (1998) Essentiality of copper in humans. Am J Clin Nutr 67:952S–959S. doi:10.1111/j.1753-4887.1987.tb06081.x
Broderius M, Prohaska JR (2009) Differential impact of copper deficiency in rats on blood cuproproteins. Nutr Res 29:494–502. doi:10.1016/j.nutres.2009.06.006
Beshgetoor D, Hambidge M (1998) Clinical conditions altering copper metabolism in humans. Am J Clin Nutr 67(suppl):1017S–1021S
Megahed MA, Hassanin KMA, Youssef IMI, Elfghi ABA, Amin KA (2013) Alterations in plasma lipids, glutathione and homocysteine in relation to dietary copper in rats. J Investig Biochem. doi:10.5455/jib.20130716075753
Aigner E, Strasser M, Haufe H, Sonnweber T, Hohla F, Stadlmayr A, Solioz M, Tilg H, Patsch W, Weiss G, Stickel F, Datz C (2010) A role for low hepatic copper concentrations in nonalcoholic fatty liver disease. Am J Gastroenterol 105(9):1978–1985. doi:10.1038/ajg.2010.170
Klevay LM, Inman L, Johnson LK, Lawler M, Mahalko JR, Milne DB, Lukaski HC, Bolonchuk W, Sandsteadet HH (1984) Increased cholesterol in plasma in a young man during experimental copper depletion. Metabolism 33:1112–1118. doi:10.1016/0026-0495(84)90096-9
Salama R, Nassar A, Nafady A, Mohamed H (2007) A novel therapeutic drug (copper nicotinic acid complex) for non-alcoholic fatty liver. Liver Int 27(4):454–464
Allen KGD, Klevay LM (1978) Copper deficiency and cholesterol metabolism in the rat. Atherosclerosis 31:259–271. doi:10.1016/0021-9150(78)90062-X
Chiou PWS, Chen CL, Chen KL, Wu CP (1999) Effect of high dietary copper on the morphology of gastro-intestinal tract in broiler chickens. Asian-Australas J Anim Sci 12:548–553. doi:10.5713/ajas.1999.548
Fry RS, Ashwell MS, Lloyd KE, O'Nan AT, Flowers WL, Stewart KR, Spears JW (2012) Amount and source of dietary copper affects small intestine morphology, duodenal lipid peroxidation, hepatic oxidative stress, and mRNA expression of hepatic copper regulatory proteins in weanling pigs. J Anim Sci 90:3112–3119. doi:10.2527/jas.2011-4403
National Research Council (1998) Nutrient requirements of swine, 10th edn. Natl. Acad. Press, Washington
Han X-Y, Du W-L, Huang Q-C, Xu Z-R, Wang Y-Z (2012) Changes in small intestinal morphology and digestive enzyme activity with oral administration of copper-loaded chitosan nanoparticles in rats. Biol Trace Elem Res 145:355–360. doi:10.1007/s12011-011-9191-x
Millsa CF, Dalgarnoa AC, Wenhama G (1976) Biochemical and pathological changes in tissues of Friesian cattle during the experimental induction of copper deficiency. Br J Nutr 35:309–331. doi:10.1079/BJN19760039
Greenwood-Van Meerveld B, Prodan CI (2012) Abnormal intestinal function related to hypocupremia in a rodent model. Neurogastroenterol Motil 24:283–287. doi:10.1111/j.1365-2982.2011.01849.x
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest. All authors have read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Tomaszewska, E., Dobrowolski, P. & Kwiecień, M. Intestinal Alterations, Basal Hematology, and Biochemical Parameters in Adolescent Rats Fed Different Sources of Dietary Copper. Biol Trace Elem Res 171, 185–191 (2016). https://doi.org/10.1007/s12011-015-0522-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-015-0522-1