Abstract
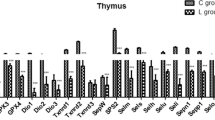
Dietary selenium (Se) deficiency induces muscular dystrophy in chicken, but the molecular mechanism remains unclear. The aim of the present study was to investigate the effect of dietary Se deficiency on the expressions of 25 selenoproteins. One-day-old broiler chickens were fed either an Se deficiency diet (0.033 mg Se/kg; produced in the Se-deficient area of Heilongjiang, China) or a diet supplemented with Se (as sodium selenite) at 0.2 mg/kg for 55 days. Then, the mRNA levels of 25 selenoproteins in chicken muscles were examined, and the principal component was further analyzed. The results showed that antioxidative selenoproteins especially Gpxs and Sepw1 were highly and extensively expressed than other types of selenoproteins in chicken muscles. In 25 selenoproteins, Gpxs, Txnrd2, Txnrd 3, Dio1, Dio 3, Selk, Sels, Sepw1, Selh, Sep15, Selu, Selpb, Sepp1, Selo, Sepx1, and SPS2 were downregulated (P < 0.05), and other selenoproteins were not influenced (P > 0.05). Se deficiency decreased the expressions of 19 selenoproteins (P < 0.05), 11 of which were antioxidative selenoproteins. And, principal component analysis (PCA) further indicated that antioxidative selenoproteins, especially Gpx3, Gpx4, and Sepw1, may play crucial roles in chicken muscles. However, compared with these antioxidative selenoproteins, some other lower expressed selenoproteins (Dio1, Selu, Selpb, Sepp1) were excessively decreased (more than 60 %, P < 0.05) by Se deficiency. Thus, it may save the limited Se levels and be beneficial to remain the level of some crucial selenoproteins. These results suggested that Se deficiency mainly influenced the expressions of antioxidative selenoproteins in chicken muscles. And, antioxidative selenoproteins especially Gpxs and Sepw1 may play a crucial role in chicken muscles. Thus, it helps us focus on some specific selenoproteins when studying the role of Se in chicken muscles.


Similar content being viewed by others
References
Rederstorff M, Krol A, Lescure A (2006) Understanding the importance of selenium and selenoproteins in muscle function. Cell Mol Life Sci 63:52–59
Yao HD, Wu Q, Zhang ZW et al (2013) Gene expression of endoplasmic reticulum resident selenoproteins correlates with apoptosis in various muscles of se-deficient chicks. J Nutr 143:613–619
Huang JQ, Li DL, Zhao H et al (2011) The selenium deficiency disease exudative diathesis in chicks is associated with downregulation of seven common selenoprotein genes in liver and muscle. J Nutr 141:1605–1610
Xu S-W, Yao H-D, Zhang J et al (2012) The oxidative damage and disbalance of calcium homeostasis in brain of chicken induced by selenium deficiency. Biol Trace Elem Res 151:225–233
Wu Q, Yao HD, Tan SR, et al. (2014) Possible Correlation of Selenoprotein W with Inflammation Factors in Chicken Skeletal Muscles. Biol Trace Elem Res. doi:10.1007/s12011-014-0092-7
Ghazi Harsini S, Habibiyan M, Moeini MM, Abdolmohammadi AR (2012) Effects of dietary selenium, vitamin E, and their combination on growth, serum metabolites, and antioxidant defense system in skeletal muscle of broilers under heat stress. Biol Trace Elem Res 148:322–330
Kieliszek M, Blazejak S (2013) Selenium: significance, and outlook for supplementation. Nutrition 29:713–718
Mariotti M, Ridge PG, Zhang Y et al (2012) Composition and evolution of the vertebrate and mammalian selenoproteomes. PLoS One 7:e33066
Bellinger FP, Raman AV, Reeves MA, Berry MJ (2009) Regulation and function of selenoproteins in human disease. Biochem J 422:11–22
Dikiy A, Novoselov SV, Fomenko DE et al (2007) SelT, SelW, SelH, and Rdx12 genomics and molecular insights into the functions. Biochemistry 46:6871–6882
Pappas AC, Zoidis E, Surai PF, Zervas G (2008) Selenoproteins and maternal nutrition. Comp Biochem Physiol B Biochem Mol Biol 151:361–372
Sunde RA, Raines AM, Barnes KM, Evenson JK (2009) Selenium status highly regulates selenoprotein mRNA levels for only a subset of the selenoproteins in the selenoproteome. Biosci Rep 29:329–338
Lescure A, Rederstorff M, Krol A, Guicheney P, Allamand V (2009) Selenoprotein function and muscle disease. Biochim Biophys Acta 1790:1569–1574
Hawkes WC, Alkan Z (2011) Delayed cell cycle progression from SEPW1 depletion is p53- and p21-dependent in MCF-7 breast cancer cells. Biochem Biophys Res Commun 413:36–40
Hawkes WC, Alkan Z (2012) Delayed cell cycle progression in selenoprotein W-depleted cells is regulated by a mitogen-activated protein kinase kinase 4-p38/c-Jun NH2-terminal kinase-p53 pathway. J Biol Chem 287:27371–27379
Hawkes WC, Printsev I, Alkan Z (2012) Selenoprotein W depletion induces a p53- and p21-dependent delay in cell cycle progression in RWPE-1 prostate epithelial cells. J Cell Biochem 113:61–69
Martinez A, Santiago JL, Varade J et al (2008) Polymorphisms in the selenoprotein S gene: lack of association with autoimmune inflammatory diseases. BMC Genomics 9:329
Du S, Zhou J, Jia Y, Huang K (2010) SelK is a novel ER stress-regulated protein and protects HepG2 cells from ER stress agent-induced apoptosis. Arch Biochem Biophys 502:137–143
Du S, Liu H, Huang K (2010) Influence of SelS gene silence on beta-mercaptoethanol-mediated endoplasmic reticulum stress and cell apoptosis in HepG2 cells. Biochim Biophys Acta 1800:511–517
Verma S, Hoffmann FW, Kumar M et al (2011) Selenoprotein K knockout mice exhibit deficient calcium flux in immune cells and impaired immune responses. J Immunol 186:2127–2137
Holben DH, Smith AM (1999) The diverse role of selenium within selenoproteins. J Am Diet Assoc 99:836–843
Liu Y, Zhao H, Zhang Q et al (2012) Prolonged dietary selenium deficiency or excess does not globally affect selenoprotein gene expression and/or protein production in various tissues of pigs. J Nutr 142:1410–1416
Ruan H, Zhang Z, Wu Q et al (2012) Selenium regulates gene expression of selenoprotein W in chicken skeletal muscle system. Biol Trace Elem Res 145:59–65
Peirson SN, Butler JN, Foster RG (2003) Experimental validation of novel and conventional approaches to quantitative real-time PCR data analysis. Nucleic Acids Res 31:e73
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408
Messaoudi I, Banni M, Said L, Said K, Kerkeni A (2010) Involvement of selenoprotein P and GPx4 gene expression in cadmium-induced testicular pathophysiology in rat. Chem Biol Interact 188:94–101
Zhang J, Li J, Zhang Z et al (2012) Ubiquitous expression of selenoprotein N transcripts in chicken tissues and early developmental expression pattern in skeletal muscles. Biol Trace Elem Res 146:187–191
Gao X, Xing H, Li S et al (2012) Selenium regulates gene expression of selenoprotein W in chicken gastrointestinal tract. Biol Trace Elem Res 145:181–188
Sun B, Wang R, Li J, Jiang Z, Xu S (2011) Dietary selenium affects selenoprotein W gene expression in the liver of chicken. Biol Trace Elem Res 143:1516–1523
Wang R, Sun B, Zhang Z, Li S, Xu S (2011) Dietary selenium influences pancreatic tissue levels of selenoprotein W in chickens. J Inorg Biochem 105:1156–1160
Yu D, Li JL, Zhang JL, Gao XJ, Xu S (2011) Effects of dietary selenium on selenoprotein W gene expression in the chicken immune organs. Biol Trace Elem Res 144:678–687
Li JL, Li HX, Li S et al (2012) Effects of Selenoprotein W gene expression by selenium involves regulation of mRNA stability in chicken embryos neurons. Biometals 25:459–468
Liu CP, Fu J, Lin SL, Wang XS, Li S (2014) Effects of dietary selenium deficiency on mRNA levels of twenty-one selenoprotein genes in the liver of layer chicken. Biol Trace Elem Res 159:192–198
Lin SL, Wang CW, Tan SR, et al. (2014) Selenium Deficiency Inhibits the Conversion of Thyroidal Thyroxine (T) to Triiodothyronine (T ) in Chicken Thyroids. Biol Trace Elem Res. doi:10.1007/s12011-014-0083-8
Liang Y, Lin SL, Wang CW et al (2014) Effect of selenium on selenoprotein expression in the adipose tissue of chickens. Biol Trace Elem Res 160:41–48
Reeves MA, Bellinger FP, Berry MJ (2010) The Neuroprotective Functions of Selenoprotein M and its Role in Cytosolic Calcium Regulation. Antioxidants & redox signaling 12(7):809–818
Wang X-L, Yang C-P, Xu K, Qin O-J (2010) Selenoprotein W depletion in vitro might indicate that its main function is not as an antioxidative enzyme. Biochem Mosc 75:201–207
Yao HD, Wu Q, Zhang ZW et al (2013) Selenoprotein W serves as an antioxidant in chicken myoblasts. Biochim Biophys Acta 1830:3112–3120
Nishida H, Ichikawa H, Konishi T (2007) Shengmai-san enhances antioxidant potential in C2C12 myoblasts through the induction of intracellular glutathione peroxidase. J Pharmacol Sci 105:342–352
Franco AA, Odom RS, Rando TA (1999) Regulation of antioxidant enzyme gene expression in response to oxidative stress and during differentiation of mouse skeletal muscle. Free Radic Biol Med 27:1122–1132
McClung JP, Roneker CA, Mu W et al (2004) Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase. Proc Natl Acad Sci U S A 101:8852–8857
Wang XD, Vatamaniuk MZ, Wang SK et al (2008) Molecular mechanisms for hyperinsulinaemia induced by overproduction of selenium-dependent glutathione peroxidase-1 in mice. Diabetologia 51:1515–1524
Hawkes WC, Alkan Z (2010) Regulation of redox signaling by selenoproteins. Biol Trace Elem Res 134:235–251
Acknowledgments
This study was supported by the National Natural Science Foundation of China (31272626); the International (Regional) Cooperation and Exchange Projects of the National Natural Science Foundation of China (31320103920); the Study Abroad Foundation of Heilongjiang Province (LC201031); and the Doctoral Fund of the Ministry of Education of China (20122325110018). We are thankful for the support of the Key Laboratory of Myocardial Ischemia, Harbin Medical University. The authors thank Elsevier English Language Editing System to correct grammatical, spelling, and other common errors.
Conflict of Interest
The authors declare that there are no conflicts of interest
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yao, H., Zhao, W., Zhao, X. et al. Selenium Deficiency Mainly Influences the Gene Expressions of Antioxidative Selenoproteins in Chicken Muscles. Biol Trace Elem Res 161, 318–327 (2014). https://doi.org/10.1007/s12011-014-0125-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-014-0125-2