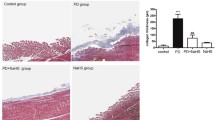
Abstract
Peritoneal fibrosis resulting from long-term clinical peritoneal dialysis has been the main reason of dropout from peritoneal dialysis. Peritonitis as a common complication of peritoneal dialysis treatment may lead to the occurrences of peritoneal fibrosis. We cultured peritoneal mesothelial cells with lipopolysaccharides (LPS) in order to stimulate the environment of peritonitis and investigate whether lipopolysaccharides could induce epithelial-to-mesenchymal transition (EMT). Oxidative stress could stimulate fibrogenesis while selenium has antioxidant properties. So, this study also explored whether selenium supplementation affects lipopolysaccharide-induced EMT and fibrosis. We found that lipopolysaccharides could activate EMT changes such as the loss of E-cadherin and the increase of α-smooth muscle actin (α-SMA), collagen I, vimentin, and fibronectin (FN), while selenium inhibits EMT by modulating reactive oxygen species (ROS) generation and ROS/MMP-9 signaling pathways in peritoneal mesothelial cells. Moreover, it was revealed that selenium decreased the EMT events of peritoneal mesothelial cells via inhibition of PI3k/AKT pathways. In conclusion, these findings enable a better understanding of the mechanism of peritoneal fibrosis and explore a new idea for the prevention and treatment.




Similar content being viewed by others
References
Zuo L, Wang M, Chinese Association of Blood Purification Management of Chinese Hospital Association (2010) Current burden and probable increasing incidence of ESRD in China. Clin Nephrol 74(Suppl 1):S20–S22
Modi GK, Jha V (2006) The incidence of end-stage renal disease in India: a population-based study. Kidney Int 70(12):2131–2133
Yang X, Yi C, Liu X et al (2013) Clinical outcome and risk factors for mortality in Chinese patients with diabetes on peritoneal dialysis: a 5-year clinical cohort study. Diabetes Res Clin Pract 100(3):354–361
Mehrotra R, Chiu YW, Kalantar-Zadeh K et al (2011) Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Arch Intern Med 171(2):110–118
Yeates K, Zhu N, Vonesh E et al (2012) Hemodialysis and peritoneal dialysis are associated with similar outcomes for end-stage renal disease treatment in Canada. Nephrol Dial Transplant 27(9):3568–3575
Ueno T, Nakashima A, Doi S et al (2013) Mesenchymal stem cells ameliorate experimental peritoneal fibrosis by suppressing inflammation and inhibiting TGF-β1 signaling. Kidney Int 84(2):297–307
Kitamoto M, Kato K, Sugimoto A et al (2011) Sairei-to ameliorates rat peritoneal fibrosis partly through suppression of oxidative stress. Nephron Exp Nephrol 117(3):e71–e81
de Lima SM, Otoni A, Sabino Ade P et al (2013) Inflammation, neoangiogenesis and fibrosis in peritoneal dialysis. Clin Chim Acta 421:46–50
Margetts PJ, Kolb M, Yu L et al (2001) A chronic inflammatory infusion model of peritoneal dialysis in rats. Perit Dial Int 21:S368–S372
Borges FR, Silva MD, Córdova MM et al (2014) Anti-inflammatory action of hydroalcoholic extract, dichloromethane fraction and steroid α-spinasterol from Polygala sabulosa in LPS-induced peritonitis in mice. J Ethnopharmacol 151(1):144–150
Liu ZW, Zhu HT, Chen KL et al (2013) Selenium attenuates high glucose-induced ROS/TLR-4 involved apoptosis of rat cardiomyocyte. Biol Trace Elem Res 156(1–3):262–270
Yang SY, Zhang L, Miao KK et al (2013) Effects of selenium intervention on chronic fluorosis-induced renal cell apoptosis in rats. Biol Trace Elem Res 153(1–3):237–242
Cases J, Vacchina V, Napolitano A et al (2001) Selenium from selenium-rich Spirulina is less bioavailable than selenium from sodium selenite and selenomethionine in selenium-deficient rats. J Nutr 131(9):2343–2350
Babaknejad N, Sayehmiri F, Sayehmiri K et al (2014) The relationship between selenium levels and breast cancer: a systematic review and meta-analysis. Biol Trace Elem Res 159(1–3):1–7
Tarp U, Overvad K, Hansen JC et al (1985) Low selenium level in severe rheumatoid arthritis. Scand J Rheumatol 14(2):97–101
Loscalzo J (2014) Keshan disease, selenium deficiency, and the selenoproteome. N Engl J Med 370(18):1756–1760
Nazıroğlu M, Yıldız K, Tamtürk B et al (2012) Selenium and psoriasis. Biol Trace Elem Res 150(1–3):3–9
Senol N, Nazıroğlu M, Yürüker V (2014) N-Acetylcysteine and selenium modulate oxidative stress, antioxidant vitamin and cytokine values in traumatic brain injury-induced rats. Neurochem Res 39(4):685–692
Nazıroğlu M, Karaoğlu A, Orhan Aksoy A (2004) Selenium and high dose vitamin E administration protects cisplatin-induced oxidative damage to renal, liver and lens tissues in rats. Toxicology 195:221–230
Ding M, Potter JJ, Liu X et al (2010) Selenium supplementation decreases hepatic fibrosis in mice after chronic carbon tetrachloride administration. Biol Trace Elem Res 133(1):83–97
Zhu X, Guo K, Lu Y (2011) Selenium effectively inhibits 1,2-dihydroxynaphthalene-induced apoptosis in human lens epithelial cells through activation of PI3-K/Akt pathway. Mol Vis 17:2019–2027
Yu H, Huang J, Wang S et al (2013) Overexpression of Smad7 suppressed ROS/MMP9-dependent collagen synthesis through regulation of heme oxygenase-1. Mol Biol Rep 40(9):5307–5314
Tobar N, Villar V, Santibanez JF (2010) ROS-NFkappaB mediates TGF-beta1-induced expression of urokinase-type plasminogen activator, matrix metalloproteinase-9 and cell invasion. Mol Cell Biochem 340(1–2):195–202
Boca M, D’Amato L, Distefano G et al (2007) Polycystin-1 induces cell migration by regulating phosphatidylinositol 3-kinase-dependent cytoskeletal rearrangements and GSK3beta-dependent cell cell mechanical adhesion. Mol Biol Cell 18:4050–4061
Tiwari N, Gheldof A, Tatari M et al (2012) EMT as the ultimate survival mechanism of cancer cells. Semin Cancer Biol 22(3):194–207
Aroeira LS, Aguilera A, Sánchez-Tomero JA et al (2007) Epithelial to mesenchymal transition and peritoneal membrane failure in peritoneal dialysis patients: pathologic significance and potential therapeutic interventions. J Am Soc Nephrol 18(7):2004–2013
Zhou Q, Yang M, Lan H et al (2013) miR-30a negatively regulates TGF-β1-induced epithelial-mesenchymal transition and peritoneal fibrosis by targeting Snai1. Am J Pathol 183(3):808–819
Yokoi H, Kasahara M, Mori K et al (2012) Pleiotrophin triggers inflammation and increased peritoneal permeability leading to peritoneal fibrosis. Kidney Int 81(2):160–169
Gangji AS, Brimble KS, Margetts PJ (2009) Association between markers of inflammation, fibrosis and hypervolemia in peritoneal dialysis patients. Blood Purif 28(4):354–358
Zhao L, Yang R, Cheng L et al (2011) LPS-induced epithelial-mesenchymal transition of intrahepatic biliary epithelial cells. J Surg Res 171(2):819–825
Lee HB, Ha H (2007) Mechanisms of epithelial-mesenchymal transition of peritoneal mesothelial cells during peritoneal dialysis. J Korean Med Sci 22(6):943–945
Yu MA, Shin KS, Kim JH et al (2009) HGF and BMP-7 ameliorate high glucose-induced epithelial-to-mesenchymal transition of peritoneal mesothelium. J Am Soc Nephrol 20(3):567–581
Copeland JW, Beaumont BW, Merrilees MJ et al (2007) Epithelial-to-mesenchymal transition of human proximal tubular epithelial cells: effects of rapamycin, mycophenolate, cyclosporin, azathioprine, and methylprednisolone. Transplantation 83(6):809–814
Fusshoeller A (2008) Histomorphological and functional changes of the peritoneal membrane during long-term peritoneal dialysis. Pediatr Nephrol 23(1):19–25
Duan SB, Liu GL, Wang YH et al (2012) Epithelial-to-mesenchymal transdifferentiation of renal tubular epithelial cell mediated by oxidative stress and intervention effect of probucol in diabetic nephropathy rats. Ren Fail 34(10):1244–1251
Baker RD, Baker SS, LaRosa K et al (1993) Selenium regulation of glutathione peroxidase in human hepatoma cell line Hep3B. Arch Biochem Biophys 304(1):53–57
Lothrop AP, Snider GW, Ruggles EL et al (2014) Why is mammalian thioredoxin reductase 1 so dependent upon the use of selenium? Biochemistry 53(3):554–565
Saito Y, Yoshida Y, Akazawa T et al (2003) Cell death caused by selenium deficiency and protective effect of antioxidants. J Biol Chem 278(41):39428–39434
Uğuz AC, Nazıroğlu M (2012) Effects of selenium on calcium signaling and apoptosis in rat dorsal root ganglion neurons induced by oxidative stress. Neurochem Res 37(8):1631–1638
Uğuz AC, Naziroğlu M, Espino J et al (2009) Selenium modulates oxidative stress-induced cell apoptosis in human myeloid HL-60 cells through regulation of calcium release and caspase-3 and -9 activities. J Membr Biol 232(1–3):15–23
Vunta H, Davis F, Palempalli UD et al (2007) The anti-inflammatory effects of selenium are mediated through 15-deoxy-Delta12,14-prostaglandin J2 in macrophages. J Biol Chem 282(25):17964–17973
Zhang W, Zhang R, Wang T et al (2014) Selenium inhibits LPS-induced pro-inflammatory gene expression by modulating MAPK and NF-κB signaling pathways in mouse mammary epithelial cells in primary culture. Inflammation 37(2):478–485
Zeng R, Yao Y, Han M et al (2008) Biliverdin reductase mediates hypoxia-induced EMT via PI3-kinase and Akt. J Am Soc Nephrol 19:380–387
Conflict of Interest
Jinyan Liu, Lingling Zeng, Yuliang Zhao, Bin Zhu, Wanjun Ren, and Chunling Wu have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, J., Zeng, L., Zhao, Y. et al. Selenium Suppresses Lipopolysaccharide-Induced Fibrosis in Peritoneal Mesothelial Cells Through Inhibition of Epithelial-to-Mesenchymal Transition. Biol Trace Elem Res 161, 202–209 (2014). https://doi.org/10.1007/s12011-014-0091-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-014-0091-8