Abstract
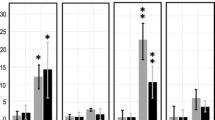
The changes in protease activities in embryonic axes during the first days of bean (Phaseolus vulgaris L.) seed germination were investigated in response to copper stress. Synthetic substrates and specific protease inhibitors have been used to define qualitatively and quantitatively different catalytic classes, particularly endoproteases (EP), carboxypeptidases (CP) and aminopeptidases (AP), then identify which ones were affected in the presence of copper. In fact, a failure in storage proteins mobilization and a disorder of nitrogen supply at enzymatic level occurred in Cu. In fact, Cu inhibited azocaseinolytic activity (ACA) and cysteine-, aspartic-, serine-, and metallo-endopeptidases activities (Cys-EP, Asp-EP, Ser-Ep, and Met-EP, respectively). Besides, Cu affected leucine- and proline-aminopeptidases (LAP and PAP, respectively) and glycine-carboxypeptidases (Gly-CP). The proteolytic responses might also be associated with the decrease in defense capacity in the Cu-treated embryos.




Similar content being viewed by others
Abbreviations
- DAN:
-
Dicyclohexylaminenitrite
- E-64:
-
l-trans-epoxysuccinyl-leucylamide-4-guanidino-butane
- FW:
-
Fresh weight
- LAP:
-
Leucine aminopeptidases
- Leu-ßNA:
-
l-leucine-ß-naphthylamide
- NEM:
-
N-ethylmaleimide
- PAP:
-
Proline aminopeptidases
- PMSF:
-
Phenylmethylsulfonylfluoride
- Pro-ßNA:
-
l-proline-ß-naphthylamide
- STI:
-
Soybean trypsin inhibitor
References
Ernst WHO (1998) Effects of heavy metals in plants at the cellular and organismic level ecotoxicology. In: Gerrit S, Bernd M III (eds) Bioaccumulation and biological effects of chemicals. Wiley and Spektrum Akademisher Verlag, Berlin, pp 587–620
Li W, Khan MA, Yamaguchi S, Kamiya Y (2005) Effects of heavy metals on seed germination and early seedling growth of Arabidopsis thaliana. Plant Growth Regul 46:45–50
Schaller A (2004) A cut above the rest: the regulatory function of plant proteases. Planta 220:183–197
Murray DR, Peoples MB, Waters SP (1979) Proteolysis in the axis of the germinating pea seed. I Changes in the protein degradation enzyme activities of the radical and the primary root. Planta 147:111–116
Murray DR, Peoples MB, Waters SP (1979) Proteolysis in the axis of the germinating pea seed. II Changes in polypeptide composition. Planta 147:117–121
Tiedemann J, Neubohn B, Müntz K (2000) Different functions of vicilin and legumin are reflected in the histopattern of globulin mobilization during germination of vetch (Vicia sativa L.). Planta 211:1–12
Schlereth A, Becker C, Horstmann C, Tiedemann J, Müntz K (2000) Comparison of globulin mobilization and cysteine proteinases in embryonic axes and cotyledons during germination and seedling growth of vetch (Vicia sativa L.). J Exp Bot 51:1423–1433
Muccifora S, Guerranti R, Muzzi CH, Hope-Onyekwere NS, Pagani R, Leoncini R, Bellani LM (2010) Ultrastructural and biochemical investigations of protein mobilization of Mucuna pruriens (L.) DC. cotyledons and embryo axis. Protoplasma 239:15–21
Alekseeva MW, Kobarskaya NB (1987) Comparative investigations of proteins from aleurone grains of embryonic axis and cotyledons of pea and soybean (russ.). Fisiol Rastenii 25:464–469
Alekseeva MW, Phoung Lien TT, Charalambous N, Jivotovskaya V (1989) Specificity of subunit composition of storage proteins in embryonic axes and cotyledons of pea seeds (russ.). Fisiol Rastenii 36:740–744
Vigil EL, Fang TK (1995) Comparative biochemical and morphological changes in imbibed cotton seed hypocotyls and radicles in situ and in vivo- protein breakdown and elongation growth. Seed Sci Res 5:41–51
Vigil EL, Fang TK (1995) Protease activities and elongation growth of excised cotton seeds during the first 24 hours of imbibition. Seed Sci Res 5:201–207
Lawrence JM, Grant DR (1963) Nitrogen mobilization in pea seedlings Il. Free amino acids. Plant Physiol 38:561–566
Rawling ND, Barrett AJ (1994) Classification of peptidases. Methods Enzymol 244:1–15
Huffaker RC (1990) Proteolytic activity during senescence of plant. New Phytol 116:199–223
Brouquisse R, Fisher A, Raymond P (1997) Proteolysis in higher plants: nature, function and regulation. In: Morot-Gaudry JF (eds) Assimilation of nitrogen in plants. Physiol biochem mol aspect, pp 327–350
Tu CJ, Park SY, Walling LL (2003) Isolation and characterization of the neutral leucine aminopeptidase (LAP-N) of tomato. Plant Physiol 132:243–255
Polge C, Jaquinod M, Holzer F, Bourguignon J, Walling L, Brouquisse R (2009) Evidence for the existence in Arabidopsis thaliana of the proteasome proteolytic pathway; activation in response to cadmium. J Biol Chem 284:35412–35424
Salt DE, Rauser WE (1995) Mg-ATP-dependent transport of phytochelatins across the tonoplast of roots. Plant Physiol 170:1293–1301
Ahsan N, Lee DG, Lee SH, Kang KY, Lee JJ, Kim PJ, Yoon HS, Kim JS, Lee BH (2007) Excess copper induced physiological and proteomic changes in germinating rice seeds. Chemosphere 67:1182–1193
Djebali W, Gallusci P, Polge C, Boulila L, Galtier N, Raymond P, Chaibi W, Brouquisse R (2007) Modifications in endopeptidase and 20S proteasome expression and activities in cadmium treated tomato (Solanum lycopersicum L.) plants. Planta 227:625–639
Domash VI, Sharpio TP, Zabreiko SA, Sosnovskaya TF (2008) Proteolytic enzymes and trypsin inhibitors of higher plants under stress conditions. Russ J Bioorg Chem 34:318–322
Sfaxi-Bousbih A, Chaoui A, El Ferjani E (2010) Unsuitable availability of nutrients in germinating bean embryos exposed to copper excess. Biol Trace Elem Res 135:295–303
Karmous I, El Ferjani E, Chaoui A (2011) Copper excess impairs mobilization of storage proteins in bean cotyledons. Biol Trace Elem Res 144:1251–1259
Karmous I, Jaouani K, Chaoui A, El Ferjani E (2012) Proteolytic activities in Phaseolus vulgaris cotyledons under copper stress. Physiol Mol Biol Plants 18:337–343
Bishnoi NR, Sheroran IS, Singh R (1993) Effect of cadmium and nickel on mobilization of food reserves and activities of hydrolytic enzymes in germinating pigeon pea seeds. Biol Plant 35:583–589
Shah K, Dubey RS (1997) Effect of cadmium on proteins, amino acids and protease, aminopeptidase and carboxypeptidase in rice seedlings. Plant Physiol Biochem 33:577–584
Kuriakose SV, Prasad MNV (2008) Cadmium stress affects seed germination and seedling growth in Sorghum bicolor (L.) Moench by changing the activities of hydrolyzing enzymes. Plant Growth Regul 54:143–156
Van Assche F, Clijsters H (1990) Effects of metals on enzyme activity in plants. Plant Cell Environ 13:195–206
Palma JM, Sandalio LM, Javier Corpas F, Romero-Puertas MC, McCarthy I, Del Rio LA (2002) Plant proteases, protein degradation, and oxidative stress: role of peroxisomes. Plant Physiol Biochem 40:521–530
Sharma SS, Dietz KJ (2009) The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci 14:43–50
Stadtman ER (1993) Oxidation of free amino acids and amino acid residues in proteins by radiolysis and by metal-catalyzed reactions. Annu Rev Biochem 62:797–891
Davies KJA (2001) Degradation of oxidized proteins by the 20 S proteasome. Biochimie 83:301–310
Müntz K (1996) Proteases and proteolytic cleavage of storage proteins in developing and germinating dicotyledonous seeds. J Exp Bot 47:605–622
Voigt G, Biehl B, Heinrichs H, Voigt J (1997) Aspartic proteinase levels in seeds of different angiosperms. Phytochemistry 444:389–392
Sutoh K, Kato H, Minamikawa T (1999) Identification and possible roles of three types of endopeptidase from germinated wheat seeds. J Biochem 126:700–707
Mutlu A, Gal S (1999) Plant aspartic proteinases: enzymes on the way to a function. Physiol Plant 105:569–576
Nguyen CV, Bielawski W, Kaczkowski J (1995) Distribution of endopeptidases in germinating triticale grains susceptible and resistant to pre-harvest sprouting. Acta Physiol Plant 17:9–16
Nielsen S, Liener IE (1984) Degradation of the major storage protein of Phaseolus vulgaris during germination: role of endogenous proteases and protease inhibitors. Plant Physiol 74:494–498
Shutov AD, Vaintraub IA (1987) Degradation of storage proteins in germinating seeds. Phytochemistry 26:1557–1566
Belozersky MA, Dunaevsky YE, Voskoboynikova E (1990) Isolation and properties of a metalloproteinase from buckwheat (Fagopyrum esculentum) seeds. Biochem J 272:677–682
Karmous I, Chaoui A, Jaouani K, Sheehan D, El Ferjani E, Scoccianti V, Crinelli R (2014) Role of the ubiquitin-proteasome pathway and some peptidases during seed germination and copper stress in bean cotyledons. Plant Physiol Biochem 76:77–85
Mikkonen A (1986) Activities of some peptidases and proteinases in germinating kidney bean Phaseolus vulgaris. Physiol Plant 68:282–286
Preston KR, Kruger JE (1986) Mobilization of monocot protein reserves during germination. In: Dalling MJ (ed) Plant proteolytic enzymes. Vol. 1. CRC Press, Boca Raton, pp 1–18
Dietz KJ, Brune A, Pfanz H (1992) Transtonoplast-transport of the sulfur-containing compounds sulfate, cysteine, methionine and glutathione. Phyton Austria 32:37–40
Wolf AE, Dietz KJ, Schrӧder P (1996) Degradation of glutathione S-conjugates by a carboxypeptidase in the plant vacuole. FEBS Lett 384:31–34
Mehta RA, Warmbardt RD, Mattoo AK (1996) Tomato fruit carboxypeptidase; properties, induction upon wounding, and immunocytochemical localization. Plant Physiol 110:883–892
Boulila-Zoghlami L, Gallusci P, Holzer FM, Basset GJ, Djebali W, Chaïbi W, Walling LL, Brouquisse R (2011) Up-regulation of leucine aminopeptidase-A in cadmium-treated tomato roots. Planta 234:4857–4863
Lomate PR, Hivrale VK (2011) Induction of leucine aminopeptidase (LAP) like activity with wounding and methyl jasmonate in pigeon pea (Cajanas cajan) suggests the role of these enzymes in plant defense in leguminosae. Plant Physiol Biochem 49:6609–6616
Lomate PR, Hivrale VK (2011) Changes and induction of aminopeptidase activities in response to pathogen infection during germination of pigeon pea (Cajanas cajan) seeds. J Plant Physiol 168:1735–1742
Kitazono A, Ito K, Yoshimoto T (1994) Prolyl aminopeptidase is not a sulfhydryl enzyme: identification of the active serine residue by site-directed mutagenesis. J Biochem 116:943–945
Acknowledgments
This work was financed by the Tunisian Ministry of Higher Education and Scientific Research (UR/11/ES-32).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karmous, I., Jaouani, K., El Ferjani, E. et al. Responses of Proteolytic Enzymes in Embryonic Axes of Germinating Bean Seeds under Copper Stress. Biol Trace Elem Res 160, 108–115 (2014). https://doi.org/10.1007/s12011-014-0020-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-014-0020-x