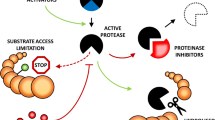
Abstract
Proteolytic enzymes are intricately involved in many aspects of plant physiology and development. On the one hand, they are necessary for protein turnover. Degradation of damaged, misfolded and potentially harmful proteins provides free amino acids required for the synthesis of new proteins. Furthermore, the selective breakdown of regulatory proteins by the ubiquitin/proteasome pathway controls key aspects of plant growth, development, and defense. Proteases are, on the other hand, also responsible for the post-translational modification of proteins by limited proteolysis at highly specific sites. Limited proteolysis results in the maturation of enzymes, is necessary for protein assembly and subcellular targeting, and controls the activity of enzymes, regulatory proteins and peptides. Proteases are thus involved in all aspects of the plant life cycle ranging from the mobilization of storage proteins during seed germination to the initiation of cell death and senescence programs. This article reviews recent findings for the major catalytic classes, i.e. the serine, cysteine, aspartic, and metalloproteases, emphasizing the regulatory function of representative enzymes.

Similar content being viewed by others
Abbreviations
- ale1 :
-
Abnormal leaf shape 1
- AP :
-
Aspartic protease
- cdr1 :
-
Constitutive disease resistance 1
- CYS-EP :
-
Cysteine endopeptidase
- LAP :
-
Leucine aminopeptidase
- PCD :
-
Programmed cell death
- PSV :
-
Protein storage vacuole
- SCP :
-
Serine carboxypeptidase
- sdd1 :
-
Stomatal density and distribution 1
- SH-EP :
-
Sulfhydryl endopeptidase
- SLD :
-
Saposin-like domain
- SPP :
-
Stromal processing peptidase
- VPE :
-
Vacuolar processing enzyme
- Zn-MP :
-
Zinc metalloprotease
References
Adam Z, Clarke AK (2002) Cutting edge of chloroplast proteolysis. Trends Plant Sci 7:451–460
Adam Z, Adamska I, Nakabayashi K, Ostersetzer O, Haussuhl K, Manuell A, Zheng B, Vallon O, Rodermel SR, Shinozaki K, Clarke AK (2001) Chloroplast and mitochondrial proteases in Arabidopsis. A proposed nomenclature. Plant Physiol 125:1912–1918
Ahn J-W, Kim M, Lim JH, Kim G-T, Pai H-S (2004) Phytocalpain controls the proliferation and differentiation fates of cells in plant organ development. Plant J 38:969–981
An C-I, Fukusaki E-I, Kobayashi A (2002) Aspartic proteinases are expressed in pitchers of the carnivorous plant Nepenthes alata Blanco. Planta 214:661–667
Asboth B, Stokum E, Khan IU, Polgar L (1985) Mechanism of action of cysteine proteinases: oxyanion binding site is not essential in the hydrolysis of specific substrates. Biochemistry 24:606–609
Athauda SB, Matsumoto K, Rajapakshe S, Kuribayashi M, Kojima M, Kubomura-Yoshida N, Iwamatsu A, Shibata C, Inoue H, Takahashi K (2004) Enzymic and structural characterization of nepenthesin, a unique member of a novel subfamily of aspartic proteinases. Biochem J 381:295–306
Bachmair A, Novatchkova M, Potuschak T, Eisenhaber F (2001) Ubiquitylation in plants: a post-genomic look at a post-translational modification. Trends Plant Sci 6:463–470
Barr PJ (1991) Mammalian subtilisins: the long-sought dibasic processing endoproteases. Cell 66:1–3
Bartling D, Nosek J (1994) Molecular and immunological characterization of leucine aminopeptidase in Arabidopsis thaliana: a new antibody suggests a semi-constitutive regulation of a phylogenetically old enzyme. Plant Sci 99:199–209
Bartling D, Weiler EW (1992) Leucine aminopeptidase from Arabidopsis thaliana. Molecular evidence for a phylogenetically conserved enzyme of protein turnover in higher plants. Eur J Biochem 205:425–431
Batchelor AK, Boutilier K, Miller SS, Labbe H, Bowman L, Hu M, Johnson DA, Gijzen M, Miki BL (2000) The seed coat-specific expression of a subtilisin-like gene, SCS1, from soybean. Planta 211:484–492
Becraft PW, Asuncion-Crabb Y (2000) Positional cues specify and maintain aleurone cell fate in maize endosperm development. Development 127:4039–4048
Becraft PW, Li K, Dey N, Asuncion-Crabb Y (2002) The maize dek1 gene functions in embryonic pattern formation and cell fate specification. Development 129:5217–5225
Beers EP, Woffenden BJ, Zhao C (2000) Plant proteolytic enzymes: possible roles during programmed cell death. Plant Mol Biol 44:399–415
Beers EP, Jones AL, Dickermann AW (2004) The S8 serine, C1A cysteine and A1 aspartic protease families in Arabidopsis. Phytochemistry 65:43–58
Belenghi B, Acconcia F, Trovato M, Perazzolli M, Bocedi A, Polticelli F, Ascenzi P, Delledonne M (2003) AtCYS1, a cystatin from Arabidopsis thaliana, suppresses hypersensitive cell death. Eur J Biochem 270:2593–2604
Belenghi B, Salomon M, Levine A (2004) Caspase-like activity in the seedlings of Pisum sativum eliminates weaker shoots during early vegetative development by induction of cell death. J Exp Bot 55:889–897
Berger D, Altmann T (2000) A subtilisin-like serine protease involved in the regulation of stomatal density and distribution in Arabidopsis thaliana. Genes Devel 14:1119–1131
Bhushan S, Lefebvre B, Ståhl A, Wright SJ, Bruce BD, Boutry M, Glaser E (2003) Dual targeting and function of a protease in mitochondria and chloroplasts. EMBO Rep 4:1073–1078
Bogacheva AM (1999) Plant subtilisins. Biochemistry (Moscow) 64:287–293
Boyd PM, Barnaby N, Tan-Wilson A, Wilson KA (2002) Cleavage specificity of the subtilisin-like protease C1 from soybean. Biochim Biophys Acta 1596:269–282
Callis J (1995) Regulation of protein degradation. Plant Cell 7:845–857
Cercos M, Urbez C, Carbonell J (2003) A serine carboxypeptidase gene (PsCP), expressed in early stages of reproductive and vegetative development in Pisum sativum, is induced by gibberellins. Plant Mol Biol 51:165–174
Chao WS, Gu Y-Q, Pautot V, Bray E, Walling LL (1999) Leucine aminopeptidase RNAs, proteins, and activities increase in response to water deficit, salinity, and the wound signals systemin, methyl jasmonate, and abscisic acid. Plant Physiol 120:979–992
Chao WS, Pautot V, Holzer FM, Walling LL (2000) Leucine aminopeptidases: the ubiquity of LAP-N and the specificity of LAP-A. Planta 210:563–573
Chen F, Foolad MR (1997) Molecular organization of a gene in barley which encodes a protein similar to aspartic protease and its specific expression in nucellar cells during degeneration. Plant Mol Biol 35:821–831
Chen G-H, Huang L-T, Yap M-N, Lee R-H, Huang Y-J, Cheng M-C, Chen S-CG (2002a) Molecular characterization of a senescence-associated gene encoding cysteine proteinase and its gene expression during leaf senescence in sweet potato. Plant Cell Physiol 43:984–991
Chen X, Pfeil JE, Gal S (2002b) The three typical aspartic proteinase genes of Arabidopsis thaliana are differentially expressed. Eur J Biochem 269:4675–4684
Chichkova NV, Kim SH, Titova ES, Kalkum M, Morozov VS, Rubtsov YP, Kalinina NO, Taliansky ME, Vartapetian AB (2004) A plant-caspase-like protease activated during hypersensitive response. Plant Cell 16:157–171
Clem RJ, Fechheimer M, Miller LK (1991) Prevention of apoptosis by a baculovirus gene during infection of insect cells. Science 254:1388–1390
Coffeen WC, Wolpert TJ (2004) Purification and characterization of serine proteases that exhibit caspase-like activity are associated with programmed cell death in Avena sativa. Plant Cell 16:857–873
Dal Degan F, Rocher A, Cameron-Mills V, von Wettstein D (1994) The expression of serine carboxypeptidases during maturation and gemination of the barley grain. Proc Natl Acad Sci USA 91:8209–8213
Danon A, Rotari VI, Gordon A, Mailhac N, Gallois P (2004) Ultraviolet-C overexposure induces programmed cell death in Arabidopsis, which is mediated by caspase-like activities and which can be suppressed by caspase inhibitors, p35 and Defender against apoptopic death. J Biol Chem 279:779–787
del Pozo O, Lam E (2003) Expression of the byculovirus p35 protein in tobacco affects cell death progression and compromises N gene mediated disease resistance response to tobacco mosaic virus. Mol Plant Microbe Interact 16:485–494
Dharmasiri N, Estelle M (2004) Auxin signaling and regulated protein degradation. Trends Plant Sci 6:302–308
Domínguez F, Cejudo FJ (1998) Germination-related genes encoding proteolytic enzymes are expressed in the nucellus of developing wheat grains. Plant J 15:569–574
Domínguez F, González MC, Cejudo FJ (2002) A germination-related gene encoding a serine carboxypeptidase is expressed during the differentiation of the vascular tissue in wheat grains and seedlings. Planta 215:727–734
Drenth J, Jansonius JN, Koekoek R, Swen HM, Wolters BG (1968) Structure of papain. Nature 218:929–932
Egas C, Lavoura N, Resende R, Brito RMM, Pires E, Pedroso de Lima MC, Faro C (2000) The saposin-like domain of the plant aspartic proteinase precursor is a potent inducer of vesicle leakage. J Biol Chem 275:38190–38196
Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S (1998) A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 391:43–50
Fisher AJ, dela Cruz W, Zoog SJ, Schneider CL, Friesen PD (1999) Crystal structure of baculovirus P35: role of a novel reactive site loop in apoptotic caspase inhibition. EMBO J 18:2031–2039
Fontanini D, Jones BL (2002) SEP-1—a subtilisin-like serine endopeptidase from germinated seeds Hordeum vulgare L. cv. Morex. Planta 215:885–893
Frigerio L, Pastres A, Prada A, Vitale A (2001) Influence of KDEL on the fate of trimeric or assembly-defective phaseolin: selective use of an alternative route to vacuoles. Plant Cell 13:1109–1126
Frugis G, Chua N-H (2002) Ubiquitin-mediated proteolysis in hormone signal transduction. Trends Cell Biol 12:308–311
Fujinaga M, Cherney MM, Oyama H, Oda K, James MNG (2004) The molecular structure and catalytic mechanism of a novel carboxyl peptidase from Scytalidium lignicolum. Proc Natl Acad Sci USA 101:3364–3369
Fuller RS, Sterne RE, Thorner J (1988) Enzymes required for yeast prohormone processing. Annu Rev Physiol 50:345–362
Funk V, Kositsup B, Zhao C, Beers EP (2002) The Arabidopsis xylem peptidase XCP1 is a tracheary element vacuolar protein that may be a papain ortholog. Plant Physiol 128:84–94
Gietl C, Schmid M (2001) Ricinosomes: an organelle for developmentally regulated programmed cell death in senescing plant tissues. Naturwissenschaften 88:49–58
Golldack D, Popova OV, Dietz K-J (2002) Mutation of the matrix metalloproteinase At2-MMP inhibits growth and causes late flowering, and early senescence in Arabidopsis. J Biol Chem 277:5541–5547
Golldack D, Vera P, Dietz KJ (2003) Expression of subtilisin-like proteases in Arabidopsis thaliana is cell specific and responds to jasmonic acid and heavy metals with developmental differences. Physiol Plant 118:64–73
Granat SJ, Wilson KA, Tan-Wilson AL (2003) New serine carboxypeptidases in mung bean seedling cotyledons. J Exp Bot 160:1263–1266
Gruis DF, Selinger DA, Curran JM, Jung R (2002) Redundant proteolytic mechanisms process seed storage proteins in the absence of seed-type members of the vacuolar processing enzyme family of cysteine proteases. Plant Cell 14:2863–2882
Gruis DF, Schulze J, Jung R (2004) Storage protein accumulation in the absence of the vacuolar processing enzyme family of cysteine proteases. Plant Cell 16:270–290
Gu Y-Q, Walling LL (2000) Specificity of the wound-induced leucine aminopeptidase (LAP-A) of tomato: activity on dipeptide and tripeptide substrates. Eur J Biochem 267:1178–1187
Gu Y-Q, Walling LL (2002) Identification of residues critical for activity of the wound-induced leucine aminopeptidase (LAP-A) of tomato. Eur J Biochem 269:1630–1640
Gu Y-Q, Holzer FM, Walling LL (1999) Overexpression, purification and biochemical characterization of the wound-induced leucine aminopeptidase of tomato. Eur J Biochem 263:726–735
Hamilton JMU, Simpson DJ, Hyman SC, Ndimba BK, Slabas AR (2003) Ara12 subtilisin-like protease from Arabidopsis thaliana: purification, substrate specificity and tissue localization. Biochem J 370:57–67
Hara-Nishimura I, Takeuchi Y, Nishimura M (1993) Molecular characterization of a vacuolar processing enzyme related to a putative cysteine proteinase of Schistosoma mansoni. Plant Cell 5:1651–1659
Hauser F, Strassner J, Schaller A (2001) Cloning, expression, and characterization of tomato (Lycopersicon esculentum) aminopeptidase P. J Biol Chem 276:31732–31737
Herbers K, Prat S, Willmitzer L (1994) Functional analysis of a leucine aminopeptidase from Solanum tuberosum L. Planta 194:230–240
Hildmann T, Ebneth M, Peña-Cortés H, Sánchez-Serrano JJ, Willmitzer L, Prat S (1992) General roles for abscisic and jasmonic acids in gene activation as a result of mechanical wounding. Plant Cell 4:1157–1170
Hoeberichts FA, Woltering EJ (2002) Multiple mediators of plant programmed cell death: interplay of conserved cell death mechanisms and plant-specific regulators. BioEssays 25:47–57
Hoeberichts FA, ten Have A, Woltering EJ (2003) A tomato metacaspase gene is upregulated during programmed cell death in Botrytis cinerea-infected leaves. Planta 217:517–522
Huffaker RC (1990) Proteolytic activity during senescence of plants. New Phytol 116:199–231
Ishii S (1994) Legumain: asparaginyl endopeptidase. Methods Enzymol 244:604–615
Janzik I, Macheroux P, Amrhein N, Schaller A (2000) LeSBT1, a subtilase from tomato plants: overexpression in insect cells, purification and characterization. J Biol Chem 275:5193–5199
Jiang L, Rogers JC (1999) Functional analysis of a Golgi-localized kex2p-like protease in tobacco suspension culture cells. Plant J 18:23–32
Jordá L, Coego A, Conejero V, Vera P (1999) A genomic cluster containing four differentially regulated subtilisin-like processing protease genes in tomato plants. J Biol Chem 274:2360–2365
Kaneda M, Tominaga N (1975) Isolation and characterization of a proteinase from the sarcocarp of melon fruit. J Biochem 78:1287–1296
Kato H, Minamikawa T (1996) Identification and characterization of a rice cysteine endoproteinase that digests glutelin. Eur J Biochem 239:310–316
Kato H, Sutoh K, Minamikawa T (2003) Identification, cDNA cloning and possible roles of seed-specific rice asparaginyl endopeptidase, REP2. Planta 217:676–685
Kervinen J, Tobin GJ, Costa J, Waugh DS, Wlodawer A, Zdanov A (1999) Crystal structure of plant aspartic proteinase prophytepsin: inactivation and vacuolar targeting. EMBO J 14:3947–3955
Kinal H, Park C-M, Berry JO, Koltin Y, Bruenn JA (1995) Processing and secretion of a virally encoded antifungal toxin in transgenic tobacco plants: evidence for a Kex2p pathway in plants. Plant Cell 7:677–688
Kinoshita T, Nishimura M, Hara-Nishimura I (1995) The sequence and expression of the gamma-VPE gene, one member of a family of three genes for vacuolar processing enzymes in Arabidopsis thaliana. Plant Cell Physiol 36:1555–1562
Kinoshita T, Yamada K, Hiraiwa N, Kondo M, Nishimura M, Hara-Nishimura I (1999) Vacuolar processing enzyme is up-regulated in the lytic vacuoles of vegetative tissues during senescence and under various stressed conditions. Plant J 19:43–53
Konno K, Hirayama C, Nakamura M, Tateishi K, Tamura Y, Hattori M, Kohno K (2004) Papain protects papaya trees from herbivorous insects: role of cysteine proteases in latex. Plant J 37:370–378
Krüger J, Thomas CM, Golstein C, Dixon MS, Smoker M, Tang S, Mulder L, Jones JDG (2002) A tomato cysteine protease required for Cf-2-dependent disease resistance and suppression of autonecrosis. Science 296:744–747
Kurochkin IV (2001) Insulin-degrading enzyme: embarking on amyloid destruction. Trends Biochem Sci 26:421–425
Kuroda H, Maliga P (2003) The plastid clpP1 protease gene is essential for plant development. Nature 425:86–89
Lam E, del Pozo O (2000) Caspase-like protease involvement in the control of plant cell death. Plant Mol Biol 44:417–428
Lehfeldt C, Shirley AM, Meyer K, Ruegger MO, Cusumano JC, Viitanen PV, Strack D, Chapple C (2000) Cloning of the SNG1 gene of Arabidopsis reveals a role for a serine carboxypeptidase-like protein as an acyltransferase in secondary metabolism. Plant Cell 12:1295–1306
Li AX, Steffens JC (2000) An acyltransferase catalyzing the formation of diacylglucose is a serine carboxypeptidase-like protein. Proc Natl Acad Sci USA 97:6902–6907
Li J, Lease KA, Tax FE, Walker JC (2001) BRS1, a serine carboxypeptidase, regulates BRI1 signaling in Arabidopsis. Proc Natl Acad Sci USA 98:5916–5921
Lid SE, Gruis DF, Jung R, Lorentzen JA, Ananiev E, Chamberlin M, Niu X, Meeley R, Nichols S, Olsen O-A (2002) The defective kernel 1 (dek1) gene required for aleurone cell development in the endosperm of maize grains encodes a membrane protein of the calpain superfamily. Proc Natl Acad Sci USA 99:5460–5465
Lincoln JE, Richael C, Overduin B, Smith K, Bostock RM, Gilchrist DG (2002) Expression of the antiapoptotic baculovirus p35 gene in tomato blocks programmed cell death and provides broad-spectrum resistance to disease. Proc Natl Acad Sci USA 99:15217–15221
Maidment JM, Moore D, Murphy GP, Murphy G, Clark IM (1999) Matrix metalloproteinase homologs from Arabidopsis thaliana. Expression and activity. J Biol Chem 274:34706–34710
Margis R, Margis-Pinheiro M (2003) Phytocalpains: orthologous calcium-dependent proteinases. Trends Plant Sci 8:58–62
Mehta RA, Warmbrandt RD, Mattoo AK (1996) Tomato fruit carboxypeptidase (properties, induction upon wounding, and immunocytochemical localization). Plant Physiol 110:883–892
Meichtry J, Amrhein N, Schaller A (1999) Characterization of the subtilase gene family in tomato (Lycopersicon esculentum Mill.). Plant Mol Biol 39:749–760
Milkowski C, Strack D (2004) Serine carboxypeptidase-like acyltransferases. Phytochemistry 65:517–524
Milkowski C, Baumert A, Schmidt D, Nehlin L, Strack D (2004) Molecular regulation of sinapate ester metabolism in Brassica napus: expression of genes, properties of the encoded proteins and correlation of enzyme activities with metabolite accumulation. Plant J 38:80–92
Moberg P, Ståhl A, Bhushan S, Wright SJ, Eriksson A, Bruce BD, Glaser E (2003) Characterization of a novel zinc metalloprotease involved in degrading targeting peptides in mitochondria and chloroplasts. Plant J 36:616–628
Mollenhauer HH, Totten C (1970) Studies on seeds. V. Microbodies, glyoxysomes, and ricinosomes of castor bean endosperm. Plant Physiol 46:794–799
Moura DS, Bergey DR, Ryan CA (2001) Characterization and localization of a wound-inducible type I serine-carboxypeptidase from leaves of tomato plants (Lycopersicon esculentum Mill.). Planta 212:222–230
Müntz K, Shutov AD (2002) Legumains and their functions in plants. Trends Plant Sci 7:340–344
Müntz K, Blattner FR, Shutov AD (2002) Legumains—a family of asparagine-specific cysteine endopeptidases involved in polypeptide processing and protein breakdown in plants. J Plant Physiol 159:1281–1293
Murakami S, Kondo Y, Nakano T, Sato F (2000) Protease activity of CND41, a chloroplast nucleoid DNA-binding protein, isolated from cultured tobacco cells. FEBS Lett 468:15–18
Mutlu A, Gal S (1999) Plant aspartic proteinases: enzymes on the way to a function. Phytochemistry 105:569–576
Nadeau JA, Sack FD (2002) Control of stomatal distribution on the Arabidopsis leaf surface. Science 296:1697–1700
Neuteboom LW, Ng JMY, Kuyper M, Clijdesdale OR, Hooykaas PJJ, van der Zaal BJ (1999) Isolation and characterization of cDNA clones corresponding with mRNAs that accumulate during auxin-induced lateral root formation. Plant Mol Biol 39:273–287
Noh YS, Amasino RM (1999) Identification of a promoter region responsible for the senescence-specific expression of SAG12. Plant Mol Biol 41:181–194
Okamoto T, Minamikawa T (1998) A vacuolar cysteine endopeptidase (SH-EP) that digests seed storage globulin: characterization, regulation of gene expression, and posttranslational processing. J Plant Physiol 152:675–682
Okamoto T, Yuki A, Mitsuhashi N, Minamikawa T (1999) Asparaginyl endopeptidase (VmPE-1) and autocatalytic processing synergistically activate the vacuolar cysteine proteinase (SH-EP). Eur J Biochem 264:223–232
Okamoto T, Shimada T, Hara-Nishimura I, Nishimura M, Minamikawa T (2003) C-terminal KDEL sequence of a KDEL-tailed cysteine proteinase (sulfhydryl-endopeptidase) is involved in formation of KDEL vesicle and in efficient vacuolar transport of sulfhydryl-endopeptidase. Plant Physiol 132:1892–1900
Pautot V, Holzer FM, Reisch B, Walling L (1993) Leucine aminopeptidase: an inducible component of the defense response in Lycopersicon esculentum (tomato). Proc Natl Acad Sci USA 90:9906–9910
Pautot V, Holzer FM, Chaufaux J, Walling LL (2001) The induction of tomato aminopeptidase genes (LapA) after Pseudomonas syringae pv. tomato infection is primarily a wound response triggered by coronatine. Mol Plant Microbe Interact 14:214–224
Payie KG, Tanaka T, Gal S, Yada RY (2003) Construction, expression and characterization of a chimaeric mammalian–plant aspartic proteinase. Biochem J 372:671–678
Pearce G, Strydom D, Johnson S, Ryan CA (1991) A polypeptide from tomato leaves induces wound-inducible proteinase inhibitor proteins. Science 253:895-898
Pechan T, Ye L, Chang Y, Mitra A, Lin L, Davis FM, Williams WP, Luthe DS (2000) A unique 33-kD cysteine proteinase accumulates in response to larval feeding in maize genotypes resistant to Fall Armyworm and other lepidoptera. Plant Cell 12:1031–1040
Pilon M, Weisbeek PJ, Kruijff B (1992) Kinetic analysis of translocation into isolated chloroplasts of the purified ferredoxin precursor. FEBS Lett 302:65–68
Popovic T, Puizdar V, Brzin J (2002) A novel subtilase from common bean leaves. FEBS Lett 530:163–168
Ramalho-Santos M, Veríssimo P, Cortes L, Samyn B, Van Beeumen J, Pires E, Faro C (1998) Identification and proteolytic processing of procardosin A. Eur J Biochem 225:133–138
Rawlings ND, Tolle DP, Barrett AJ (2004) MEROPS: the peptidase database. Nucleic Acids Res 32:D160–D164
Ribeiro A, Akkermans ADL, van Kammen A, Bisseling T, Pawlowski K (1995) A nodule-specific gene encoding a subtilisin-like protease is expressed in early stages of actinorrhizal nodule development. Plant Cell 7:785–794
Richter S, Lamppa GK (1998) A chloroplast processing enzyme functions as the general stromal processing peptidase. Proc Natl Acad Sci USA 95:7463–7468
Richter S, Lamppa GK (1999) Stromal processing peptidase binds transit peptides and initiates their ATP-dependent turnover. J Cell Biol 147:33–43
Richter S, Lamppa GK (2002) Determinants for removal and degradation of transit peptides of chloroplast precursor proteins. J Biol Chem 277:43888–43894
Richter S, Lamppa GK (2003) Structural properties of the chloroplast stromal processing peptidase required for its function in transit peptide removal. J Biol Chem 278:39497–39502
Riggs CD, Horsch A (1995) Molecular-cloning of an anther specific gene from tomato. Plant Physiol 108:117–117
Rojo E, Zouhar J, Carter C, Kovaleva V, Raikhel NV (2003) A unique mechanism for protein processing and degradation in Arabidopsis thaliana. Proc Natl Acad Sci USA 100:7389–7394
Rudenskaya GN, Bogdanova EA, Revina LP, Golovkin BN, Stepanov VM (1995) Macluralisin—a serine proteinase from fruits of Maclura pomifera (Raf.) Schneid. Planta 196:174–179
Runeberg-Roos P, Törmäkangas K, Östman A (1991) Primary structure of a barley grain aspartic proteinase. A plant aspartic proteinase resembling mammalian cathepsin D. Eur J Biochem 202:1021–1027
Ryan CA, Walker-Simmons MK (1981) Plant proteinases. In: Stumpf PK, Conn EE (eds) The biochemistry of plants. Academic Press, New York, pp 321–350
Sakai J, Rawson RB, Espenshade PJ, Cheng D, Seegmiller AC, Goldstein JL, Brown MS (1998) Molecular identification of the sterol-regulated luminal protease that cleaves SREBPs and controls lipid composition in animal cells. Mol Cell 2:505–514
Sarkkinen P, Kalkkinen N, Tilgmann C, Siuro J, Kervinen J, Micola L (1992) Aspartic proteinase from barley grains is related to mammalian lysosomal cathepsin D. Planta 186:317–323
Schaller A, Ryan CA (1994) Identification of a 50-kDa systemin-binding protein in tomato plasma membranes having Kex2p-like properties. Proc Natl Acad Sci USA 91:11802–11806
Schaller A, Bergey DR, Ryan CA (1995) Induction of wound response genes in tomato leaves by bestatin, an inhibitor of aminopeptidases. Plant Cell 7:1893–1898
Schlereth A, Standhardt D, Mock HP, Müntz K (2001) Stored cysteine proteinases start globulin mobilization in protein bodies of embryonic axes and cotyledons during vetch (Vicia sativa L.) seed germination. Planta 212:718–727
Schmid M, Simpson D, Kalousek F, Gietl C (1998) A cysteine endopeptidase with a C-terminal KDEL motif isolated from castor bean endosperm is a marker enzyme for the ricinosome, a putative lytic compartment. Planta 206: 466–475
Schmid M, Simpson D, Gietl C (1999) Programmed cell death in castor bean endosperm is associated with the accumulation and release of a cysteine endopeptidase from ricinosomes. Proc Natl Acad Sci USA 96:14159–14164
Schmid M, Simpson DJ, Sarioglu H, Lottspeich F, Gietl C (2001) The ricinosomes of senescing plant tissue bud from the endoplasmic reticulum. Proc Natl Acad Sci USA 98:5353–5358
Seidah NG, Chrétien M, Day R (1994) The family of subtilisin/kexin like pro-protein and pro-hormone convertases: divergent or shared functions. Biochimie 76:197–209
Seidah NG, Mowla SJ, Hamelin J, Mamarbachi AM, Benjannet S, Touré BB, Basak A, Munzer JS, Marcinkiewic J, Zhong M, Barale J-C, Lazure C, Murphy RA, Chrétien M, Marcinkiewicz M (1999) Mammalian subtilisin/kexin isozyme SKI-1: a widely expressed proprotein convertase with a unique cleavage specificity and cellular localization. Proc Natl Acad Sci USA 96:1321–1326
Seidah NG, Benjannet S, Wickham L, Marcinkiewicz J, Jasmin SB, Stifani S, Basak A, Prat A, Chrétien M (2003) The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc Natl Acad Sci USA 100:928–933
Serino G, Deng XW (2003) The COP9 signalosome: regulating plant development through control of proteolysis. Annu Rev Plant Biol 54:165–182
Shimada T, Fuji K, Tamura K, Kondo M, Nishimura M, Hara-Nishimura I (2003a) Vacuolar sorting receptor for seed storage proteins in Arabidopsis thaliana. Proc Natl Acad Sci USA 100:16095–16100
Shimada T, Yamada K, Kataoka M, Nakaune S, Koumoto Y, Kuroyanagi M, Tabata S, Kato T, Shinozaki K, Seki M, Kobayashi M, Kondo M, Nishimura M, Hara-Nishimura I (2003b) Vacuolar processing enzymes are essential for proper processing of seed storage proteins in Arabidopsis thaliana. J Biol Chem 278:32292–32299
Shirley AM, Chapple C (2003) Biochemical characterization of sinapoylglucose:choline sinapoyltransferase, a serine carboxypeptidase-like protein that functions as an acyltransferase in plant secondary metabolism. J Biol Chem 278:19870–19877
Shirley AM, McMichael CM, Chapple C (2001) The sng2 mutant of Arabidopsis is defective in the gene encoding the serine carboxypeptidase-like protein sinapoylglucose:choline sinapoyltransferase. Plant J 28:83–94
Simões I, Faro C (2004) Structure and function of plant aspartic proteinases. Eur J Biochem 271:2067–2075
Smalle J, Vierstra RD (2004) The ubiquitin 26S proteasome proteolytic pathway. Annu Rev Plant Biol 55:555–590
Solomon M, Belenghi B, Delledonne M, Menachem E, Levine A (1999) The involvement of cysteine proteases and protease inhibitor genes in the regulation of programmed cell death in plants. Plant Cell 11:431–443
Ståhl A, Moberg P, Ytterberg J, Panfilov O, Brockenhuus von Löwenhielm H, Nilsson F, Glaser E (2002) Isolation and identification of a novel mitochondrial metalloprotease (PreP) that degrades targeting presequences in plants. J Biol Chem 277:41931-41939
Steffens JC (2000) Acyltransferases in protease’s clothing. Plant Cell 12:1253–1255
Strassner J, Huet Y, Schaller A (2002) Cloning of tomato proteases by direct selection in yeast for enzymes that cleave the polypeptide wound signal systemin. In: Schmidt A, Mauch-Mani B (eds) Induced resistance in plants against insects and diseases. IOBC/wprs bulletin, vol 25(6). IOBC/wprs, Dijon, pp 159–163
Sullivan JA, Shirasu K, Deng XW (2003) The diverse roles of ubiquitin and the 26S proteasome in the life of plants. Nat Rev Genet 4:948–958
Tanaka H, Onouchi H, Kondo M, Hara-Nishimura I, Nishimura M, Machida C, Machida Y (2001) A subtilisin-like serine protease is required for epidermal surface formation in Arabidopsis embryos and juvenile plants. Development 128:4681–4689
Taylor A, Daims M, Lee J, Surgenor T (1982) Identification and quantification of leucine aminopeptidase in aged normal and cataractous human lens and ability of bovine lens LAP to cleave bovine crystallins. Eye Res 2:47–56
Taylor AA, Horsch A, Rzepczyk A, Hasenkampf CA, Riggs CD (1997) Maturation and secretion of a serine proteinase is associated with events of late microsporogenesis. Plant J 12:1261–1271
Than ME, Helm M, Simpson DJ, Lottspeich F, Huber R, Gietl C (2004) The 2.0 A crystal structure and substrate specificity of the KDEL-tailed cysteine endopeptidase functioning in programmed cell death of Ricinus communis endosperm. J Mol Biol 336:1103–1116
Törmäkangas K, Hadlington JL, Pimpl P, Hillmer S, Brandizzi F, Teeri TH, Denecke J (2001) A vacuolar sorting domain may also influence the way in which proteins leave the endoplasmic reticulum. Plant Cell 13:2021–2032
Tornero P, Conejero V, Vera P (1996) Primary structure and expression of a pathogen-induced protease (PR-P69) in tomato plants: similarity of functional domains to subtilisin-like endoproteases. Proc Natl Acad Sci USA 93:6332–6337
Toyooka K, Okamoto T, Minamikawa T (2000) Mass transport of proform of a KDEL-tailed cysteine protease (SH-EP) to protein storage vacuoles by endoplasmic reticulum-derived vesicle is involved in protein mobilization in germinating seeds. J Cell Biol 148:453–363
Uchikoba T, Yonezawa H, Kaneda M (1995) Cleavage specificity of cucumisin, a plant serine protease. J Biochem 117:1126–1130
Uren AG, O’Rourke K, Aravind L, Pisabarro MT, Seshagiri S, Koonin EV, Dixit VM (2000) Identification of paracaspases and metacaspases: two ancient families of caspase-like proteins, one of which plays a key role in MALT lymphoma. Mol Cell 6:961–967
VanderVere PS, Bennett TM, Oblong JE, Lamppa GK (1995) A chloroplast processing enzyme involved in precursor maturation shares a zinc-binding motif with a recently recognized family of metalloendopeptidases. Proc Natl Acad Sci USA 92:7177–7181
Varshavsky A (1996) The N-end rule: functions, mysteries, uses. Proc Natl Acad Sci USA 93:12142–12149
Vera P, Conejero V (1988) Pathogenesis-related proteins of tomato. P-69 as an alkaline endoproteinase. Plant Physiol 87:58–63
Vierstra RD (2003) The ubiquitin/26S proteasome pathway, the complex last chapter in the life of many plant proteins. Trends Plant Sci 8:135–142
Vigil EL (1970) Cytochemical and developmental changes in microbodies (glyoxysomes) and related organelles of castor bean endosperm. J Cell Biol 46:435–454
von Groll U, Berger D, Altmann T (2002) The subtilisin-like serine protease SDD1 mediates cell-to-cell signaling during stomatal development. Plant Cell Environ 14:1527–1539
Wagstaff C, Leverentz MK, Griffiths G, Thomas B, Chanasut U, Stead AD, Rogers HJ (2002) Cysteine protease gene expression and proteolytic activity during senescence of Alstroemeria petals. J Exp Bot 53:233–240
Walker-Simmons M, Ryan CA (1980) Isolation and properties of carboxypeptidase from leaves of wounded tomato plants. Phytochemistry 19:43–47
Wan L, Xia Q, Qiu X, Selveraj G (2002) Early stages of seed development in Brassica napus: a seed coat-specific cysteine proteinase associated with programmed cell death of the inner integument. Plant J 30:1–10
Woltering EJ, van der Bent A, Hoeberichts FA (2002) Do plant caspases exist? Plant Physiol 130:1764–1769
Xia Y, Suzuki H, Borevitz J, Blount J, Guo Z, Patel K, Dixon RA, Lamb C (2004) An extracellular aspartic protease functions in Arabidopsis disease signaling. EMBO J 223:980–988
Yamada T, Shimada H, Kondo M, Nishimura M, Hara-Nishimura I (1999) Multiple functional proteins are produced by cleaving Asn-Gln bonds of a single precursor by vacuolar processing enzyme. J Biol Chem 274:2563–2570
Yamagata H, Masuzawa T, Nagaoka Y, Ohnishi T, Iwasaki T (1994) Cucumisin, a serine protease from melon fruits, shares structural homology with subtilisin and is generated from a large precursor. J Biol Chem 269:32725–32731
Yin Y, Wu D, Chory J (2002) Plant receptor kinases: systemin receptor identified. Proc Natl Acad Sci USA 99:9090–9092
Zhao C, Johnson BJ, Kositsup B, Beers EP (2000) Exploiting secondary growth in Arabidopsis. Construction of xylem and bark cDNA libraries and cloning of three xylem endopeptidases. Plant Physiol 123:1185–1196
Zhong R, Wan J, Jin R, Lamppa GK (2003) A pea antisense gene for the chloroplast stromal processing peptidase yields seedling lethals in Arabidopsis: survivors show defective GFP import in vivo. Plant J 34:802–812
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schaller, A. A cut above the rest: the regulatory function of plant proteases. Planta 220, 183–197 (2004). https://doi.org/10.1007/s00425-004-1407-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-004-1407-2