Abstract
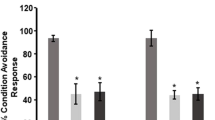
This study was aimed to investigate the effect of aluminum and extremely low-frequency magnetic fields (ELF-MF) on oxidative stress and memory of SPF Kunming mice. Sixty male SPF Kunming mice were divided randomly into four groups: control group, ELF-MF group (2 mT, 4 h/day), load aluminum group (200 mg aluminum/kg, 0.1 ml/10 g), and ELF-MF + aluminum group (2 mT, 4 h/day, 200 mg aluminum/kg). After 8 weeks of treatment, the mice of three experiment groups (ELF-MF group, load aluminum group, and ELF-MF + aluminum group) exhibited firstly the learning memory impairment, appearing that the escaping latency to the platform was prolonged and percentage in the platform quadrant was reduced in the Morris water maze (MWM) task. Secondly are the pathologic abnormalities including neuronal cell loss and overexpression of phosphorylated tau protein in the hippocampus and cerebral cortex. On the other hand, the markers of oxidative stress were determined in mice brain and serum. The results showed a statistically significant decrease in superoxide dismutase activity and increase in the levels of malondialdehyde in the ELF-MF group (P < 0.05 or P < 0.01), load aluminum group (P < 0.01), and ELF-MF + aluminum group (P < 0.01). However, the treatment with ELF-MF + aluminum induced no more damage than ELF-MF and aluminum did, respectively. In conclusion, both aluminum and ELF-MF could impact on learning memory and pro-oxidative function in Kunming mice. However, there was no evidence of any association between ELF-MF exposure with aluminum loading.









Similar content being viewed by others
Abbreviations
- ELF-MF:
-
Extremely low-frequency magnetic fields
- MWM:
-
Morris water maze
- SOD:
-
Superoxide dismutase
- MDA:
-
Malondialdehyde
- ALS:
-
Amyotrophic lateral sclerosis
- AD:
-
Alzheimer’s disease
- CNS:
-
Central nervous system
- PD:
-
Parkinson’s disease
- NFTs:
-
Neurofibrillary tangles
- PHFs:
-
Paired helical filaments
- ROS:
-
Reactive oxygen species
- CAT:
-
Catalase
- GPx:
-
Glutathione peroxidase
- PUFAs:
-
Polyunsaturated fatty acids
- EMR:
-
Electromagnetic radiation
- TBA:
-
Thiobarbituric acid
- SDS–PAGE:
-
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- PVDF:
-
Polyvinylidene fluoride
- BBB:
-
Blood–brain barrier
- PP2A:
-
Protein phosphatase 2A
- TOS:
-
Total oxidant system
- OSI:
-
Oxidative stress index
- PC:
-
Protein carbonyl
References
Ahlbom A, Adele G, Leeka K et al (2004) Epidemiology of health effects of radiofrequency exposure. Environ Health Perspect 112(17):1741–1754
Cook CM, Saucier DM, Thomas AW et al (2006) Exposure to ELF magnetic and ELF-modulated radiofrequency fields: the time course of physiological and cognitive effects observed in recent studies. Biol Electron Magn 27(8):613–627
Kafaee M, Tehranipour M, Haghpeima A et al (2010) Effects of exposure to extremely low-frequency magnetic field of 2mT intensity on spatial memory and learning in rat. Ann Gen Psychiatr 9(Suppl 1):S144
Wolf FI, Torsello A, Tedesco B et al (2005) 50-Hz extremely low frequency electromagnetic fields enhance cell proliferation and DNA damage: possible involvement of a redox mechanism. BBA Mol Cell Res 1743:120–129
Ruiz GMJ, Pena LD, Pastor JM et al (2001) 25 Hz electromagnetic field exposure has no effect on cell cycle distribution and apoptosis in U-937 and HCA-2/1cch cells. Bioelectrochemistry 53:137–140
Mcleod KJ, Collazo L (2009) Suppression of a differentiation response in MC-3T3-E1 osteoblast-like cells by sustained, low-level, 30 Hz magnetic-field exposure. Radiat Res 153:706–714
Sabine I, Alexander P, Elisabeth D et al (2005) Cell type-specific genotoxic effects of intermittent extremely low-frequency electromagnetic fields. Mutat Res 583:184–188
Zhou J, Li C, Yao G et al (2002) Gene expression of cytokine receptors in HL60 cells exposed to a 50 Hz magnetic field. Biol Electron Magn 23:339–346
Akdag MZ, Dasdag S, Cakir DU et al (2013) Do 100 and 500 μT ELF magnetic fields alter beta amyloid protein, protein carbonyl and malondialdehyde in rat brain? Electromagn Biol Med 32(3):363–372
Chu LY, Lee JH, Nam YS et al (2011) Extremely low frequency magnetic field induces oxidative stress in mouse cerebellum. Gen Physiol Biophys 30(4):415–421
Verstraeten SV, Aimo L, Oteiza PI (2008) Aluminium and lead: molecular mechanisms of brain toxicity. Arch Toxicol 82:789–802
Solfrizzi V, Colacicco AM, D'Introno A et al (2006) Macronutrients, aluminum from drinking water and foods, and other metals in cognitive decline and dementia. J Alzheimers Dis 10:303–330
Vijay K, Kiran DG (2009) Aluminium neurotoxicity: neurobehavioural and oxidative aspects. Arch Toxicol 83(11):965–978
Jesus A (2006) Tau phosphorylation and aggregation in Alzheimer’s disease pathology. FEBS Lett 580(12):2922–2927
Shimura H, Schwartz D, Gygi SP et al (2004) HIP–Hsc70 complex ubiquitinates phosphorylated tau and enhances cell survival. J Biol Chem 279:4869–4876
Nazıroğlu M (2007) New molecular mechanisms on the activation of TRPM2 channels by oxidative stress and ADP-ribose. Neurochem Res 32:1990–2001
Nazıroğlu M, Gumral N (2009) Modulator effects of selenium and L-carnitine on wireless devices (2.45 GHz) induced oxidative stress and electroencephalography records in brain of rat. Int J Radiat Biol 85:680–689
Nazıroğlu M (2012) Molecular role of catalase on oxidative stress-induced Ca (2+) signaling and TRP cation channel activation in nervous system. J Recept Signal Transduct Res 32(3):134–141
Nazıroğlu M (2009) Role of selenium on calcium signaling and oxidative stress-induced molecular pathways in epilepsy. Neurochem Res 34:2181–2191
Çelik S, Yilmaz Ö, Aşan T et al (1999) Influence of dietary selenium and vitamin E on the levels of fatty acids in brain and liver tissues of lambs. Cell Biochem Funct 17:115–121
Nazıroğlu M, Çelik Ö, Özgül C et al (2012) Melatonin modulates wireless devices (2.45 GHz)-induced brain and dorsal root ganglion injury through TRPM2 and voltage gated calcium channels in rat. Physiol Behav 105:683–692
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Alexandre L, Anne B (2006) Individual subject sensitivity to extremely low frequency magnetic field. Neurotoxicology 27(4):534–546
Miu AC, Olteanu AI, Miclea M (2004) A behavioral and ultrastructural dissection of the interference of aluminum with aging. J Alzheimers Dis 6:315–328
Gulturk S, Demirkazik A, Kosar I et al (2010) Effect of exposure to 50 Hz magnetic field with or without insulin on blood brain barrier permeability in streptozotocin-induced diabetic rats. Bioelectromagnetics 31:262–269
Walton JR (2012) Cognitive deterioration and associated pathology induced by chronic low-level aluminum ingestion in a translational rat model provides an explanation of Alzheimer's disease, tests for susceptibility and avenues for treatment. Int J Alzheimers Dis. doi:10.1155/2012/914947
Clark RE, Broadbent NJ, Squire LR (2007) The hippocampus and spatial memory: findings with a novel modification of the water maze. J Neurosci 27:6647–6654
Rudi DH, Peter PD (2001) Applications of the Morris water maze in the study of learning and memory. Behav Brain Res 36:60–90
Florian PR (2004) Hippocampal CA3-region is crucial for acquisition and memory consolidation in Morris water maze task in mice. Behav Brain Res 154:365–374
Lee I, Kesner RP (2002) Differential contribution of NMDA receptors in hippocampal subregions to spatial working memory. Nat Neurosci 5:162–168
Gong CX, Lidsky T, Wegiel J et al (2000) Phosphorylation of microtubule-associated protein tau is regulated by protein phosphatase 2A in mammalian brain. J Biol Chem 275:5535–5544
Walton JR (2007) An aluminum-based rat model for Alzheimer’s disease exhibits oxidative damage, inhibition of PP2A activity, hyperphosphorylated tau, and granulovacuolar degeneration. J Inorg Biochem 101:1275–1284
Walton JR (2009) Functional impairment in aged rats chronically exposed to human range dietary aluminum equivalents. Neurotoxicology 30:182–193
Mondragón RS, Perry G, Luna–Muñoz J et al (2013) Phosphorylation of tau protein at sites Ser396–404 is one of the earliest events in Alzheimer's disease and Down syndrome. Neuropathol Appl Neurobiol. doi:10.1111/nan.12084
Berg H (1993) Electrostimulation of cell metabolism by low frequency electric and electromagnetic fields. Bioelectrochem Bioenerg 31:1–25
Traystman RJ, Kirsch JR, Koehler RC (1991) Oxygen radical mechanisms of brain injury following ischemia and reperfusion. J Appl Physiol 71(4):1185–1195
Sámano JM, Patricia VTD, Marco AJO et al (2012) Effect of acute extremely low frequency electromagnetic field exposure on the antioxidant status and lipid levels in rat brain. Arc Med Res 43(3):183–189
Yuan CY, Lee YJ, Wang H (2012) Aluminum overload increases oxidative stress in four functional brain areas of neonatal rats. J Biomed Sci 19:51
Akdag MZ, Dasdag S, Ulukaya E et al (2010) Effects of extremely low-frequency magnetic field on caspase activities and oxidative stress values in rat brain. Biol Trace Elem Res 138:238–249
Tripathi S, Mahdi AA, Nawab A et al (2009) Influence of age on aluminum induced lipid peroxidation and neurolipofuscin in frontal cortex of rat brain: a behavioral, biochemical and ultrastructural study. Brain Res 1253:107–116
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deng, Y., Zhang, Y., Jia, S. et al. Effects of Aluminum and Extremely Low Frequency Electromagnetic Radiation on Oxidative Stress and Memory in Brain of Mice. Biol Trace Elem Res 156, 243–252 (2013). https://doi.org/10.1007/s12011-013-9847-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-013-9847-9