Abstract
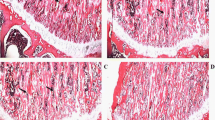
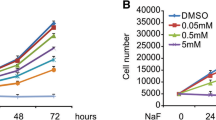
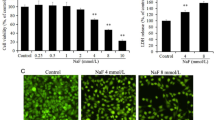
The aberrant activation of osteoblasts in the early stage is one of the critical steps during the pathogenesis of skeletal fluorosis. The endoplasmic reticulum (ER) stresses and unfolded protein response (UPR) are initiated to alleviate the accumulation of unfolded proteins against cell injury. The previous researches had demonstrated that fluoride induced ER stress in other cells or tissues. In this study, we determined the ER stress and UPR to investigate their roles in aberrant activation of fluoride-treated osteoblasts. The gene expression of bone markers and UPR factors in MC3T3-E1 cells treated with varying doses of fluoride administration was analyzed. Meantime, levels of glutathione and glutathione disulfide were tested by the ultraperformance liquid chromatography–tandem mass spectrometry applications. Our results indicated that a certain dose and period of fluoride administration induced cell proliferation and differentiation, and Runx2 was involved in the regulation of osteoblastic differentiation of MC3T3-E1 cells. Increase trend of Runx2 expression was consistent with change of marker of ER stress. Fluoride caused ER stress and stimulated UPR during the process of osteoblast maturation, while oxidative stress was also active in the occurrence of ER stress. These data indicated that ER stress and UPR were possibly involved in the action of fluoride on osteoblasts.







Similar content being viewed by others
References
Boivin G, Chavassieux P, Chapuy MC et al (1990) Skeletal fluorosis: histomorphometric findings. J Bone Miner Res Suppl 1:S185–S189
Li G, Ren L (1997) Effects of excess fluoride on bone turnover under conditions of diet with different calcium contents. Zhonghua Bing Li Xue Za Zhi 26:277–280
Nair M, Belak ZR, Ovsenek N (2011) Effects of fluoride on expression of bone-specific genes in developing Xenopus laevis larvae. Biochem Cell Biol 89:377–386
Everett ET (2011) Fluoride’s effects on the formation of teeth and bones, and the influence of genetics. J Dent Res 90:552–560
Schroder M, Kaufman RJ (2005) ER stress and the unfolded protein response. Mutat Res 569:29–63
Chakrabarti A, Chen AW, Varner JD (2011) A review of the mammalian unfolded protein response. Biotechnol Bioeng 108:2777–2793
Saito A, Ochiai K, Kondo S et al (2011) Endoplasmic reticulum stress response mediated by the PERK-eIF2(alpha)-ATF4 pathway is involved in osteoblast differentiation induced by BMP2. J Biol Chem 286:4809–4818
Jang WG, Kim EJ, Kim DK et al (2012) BMP2 protein regulates osteocalcin expression via Runx2-mediated Atf6 gene transcription. J Biol Chem 287:905–915
Tohmonda T, Miyauchi Y, Ghosh R et al (2011) The IRE1α-XBP1 pathway is essential for osteoblast differentiation through promoting transcription of osterix. EMBO Rep 12:451–457
Dequeker J, Declerck K, Schweiz MW (1993) Fluor in the treatment of osteoporosis. An overview of thirty years clinical research. Schweiz Med Wochenschr 123:2228–2234
Xu H, Jing L, Li GS (2008) Proteomic analysis of osteoblasts exposed to fluoride in vitro. Biol Trace Elem Res 123(1–3):91–7
Liu H, Sun JC, Zhao ZT (2011) Fluoride-induced oxidative stress in three-dimensional culture of OS732 cells and rats. Biol Trace Elem Res 143:446–456
Hong D, Chen HX, Yu HQ et al (2010) Morphological and proteomic analysis of early stage of osteoblast differentiation in osteoblastic progenitor cells. Exp Cell Res 316:2291–2300
Song YE, Tan H, Liu KJ et al (2011) Effect of fluoride exposure on bone metabolism indicators ALP, BALP, and BGP. Environ Health Prev Med 16:158–163
Bharti VK, Gupta M, Lall D (2008) Ameliorative effects of boron on serum profile in buffalo (Bubalus bubalis) fed high fluoride ration. Trop Anim Health Prod 40:111–116
Komori T, Yagi H, Nomura S et al (1997) Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89:755–764
Nakashima K, Zhou X, Kunkel G et al (2002) The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108:17–29
Sharma R, Tsuchiya M, Bartlett JD (2008) Fluoride induces endoplasmic reticulum stress and inhibits protein synthesis and secretion. Environ Health Perspect 116:1142–1146
Ito M, Nakagawa H, Okada T et al (2009) ER-stress caused by accumulated intracistanal granules activates autophagy through a different signal pathway from unfolded protein response in exocrine pancreas cells of rats exposed to fluoride. Arch Toxicol 83:151–159
Xu H, Zhou YL, Zhang XY et al (2010) Activation of PERK signaling through fluoride-mediated endoplasmic reticulum stress in OS732 cells. Toxicology 277:1–5
Hino S, Kondo S, Yoshinaga K et al (2010) Regulation of ER molecular chaperone prevents bone loss in a murine model for osteoporosis. J Bone Miner Metab 28:131–138
Wei J, Sheng X, Feng D et al (2008) PERK is essential for neonatal skeletal development to regulate osteoblast proliferation and differentiation. J Cell Physiol 217:693–707
Cullinan SB, Diehl JA (2006) Coordination of ER and oxidative stress signaling: the PERK/Nrf2 signaling pathway. Int J Biochem Cell Biol 38:317–32
Baird L, Dinkova-Kostova AT (2011) The cytoprotective role of the Keap1-Nrf2 pathway. Arch Toxicol 85:241–272
Acknowledgments
This work was supported by a grant for skeletal fluorosis research from the National Natural Science Foundation of China (81072249) and the Norman Bethune Program of Jilin University (2012222).
Conflict of Interest
The authors have no conflicts to disclose.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhou, Yl., Shi, Hy., Li, Xn. et al. Role of Endoplasmic Reticulum Stress in Aberrant Activation of Fluoride-Treated Osteoblasts. Biol Trace Elem Res 154, 448–456 (2013). https://doi.org/10.1007/s12011-013-9752-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-013-9752-2