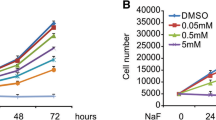
Abstract
Fluoride is a persistent environmental pollutant, and its excessive intake causes skeletal and dental fluorosis. However, few studies focused on the effects of fluoride on osteocytes, making up over 95% of all bone cells. This study aimed to investigate the effect of fluoride on osteocytes in vitro, as well as explore the underlying mechanisms. CCK-8, LDH assay, fluorescent probes, flow cytometry, and western blotting were performed to examine cell viability, apoptosis, mitochondria changes, reactive oxygen species (ROS) and mitochondrial ROS (mtROS), and protein expressions. Results showed that sodium fluoride (NaF) exposure (4, 8 mmol/L) for 24 h inhibited the cell viability of osteocytes MLO-Y4 and promoted G0/G1 phase arrest and increased cell apoptosis. NaF treatment remarkably caused mitochondria damage, loss of MMP, ATP decrease, Cyto c release, and Bax/Bcl-2 ratio increase and elevated the activity of caspase-9 and caspase-3. Furthermore, NaF significantly upregulated the expressions of LC-3II, PINK1, and Parkin and increased autophagy flux and the accumulation of acidic vacuoles, while the p62 level was downregulated. In addition, NaF exposure triggered the production of intracellular ROS and mtROS and increased malondialdehyde (MDA); but superoxide dismutase (SOD) activity and glutathione (GSH) content were decreased. The scavenger N-acetyl-L-cysteine (NAC) significantly reversed NaF-induced apoptosis and mitophagy, suggesting that ROS is responsible for the mitochondrial-mediated apoptosis and mitophagy induced by NaF exposure. These findings provide in vitro evidence that apoptosis and mitophagy are cellular mechanisms for the toxic effect of fluoride on osteocytes, thereby suggesting the potential role of osteocytes in skeletal and dental fluorosis.







Similar content being viewed by others
Data Availability
The data of this study will be made available on reasonable request.
References
Jha SK, Mishra VK, Sharma DK, Damodaran T (2011) Fluoride in the environment and its metabolism in humans. Rev Environ Contam Toxicol 211:121–142. https://doi.org/10.1007/978-1-4419-8011-3_4
Abduweli UD, Goin DE, Martinez-Mier EA, Woodruff T, DenBesten P (2020) Maternal and fetal exposures to fluoride during mid-gestation among pregnant women in northern California. Environ Health-Glob 19:38. https://doi.org/10.1186/s12940-020-00581-2
Atmaca N, Atmaca HT, Kanici A, Anteplioglu T (2014) Protective effect of resveratrol on sodium fluoride-induced oxidative stress, hepatotoxicity and neurotoxicity in rats. Food Chem Toxicol 70:191–197. https://doi.org/10.1016/j.fct.2014.05.011
Wu L, Fan C, Zhang Z, Zhang X, Lou Q, Guo N, Huang W, Zhang M, Yin F, Guan Z, Yang Y, Gao Y (2021) Association between fluoride exposure and kidney function in adults: a cross-sectional study based on endemic fluorosis area in China. Ecotoxicol Environ Saf 225:112735. https://doi.org/10.1016/j-ecoenv-2021-112735
Quadri JA, Sarwar S, Pinky Kar P, Singh S, Mallick SR, Arava S, Nag TC, Roy TS, Shariff A (2018) Fluoride induced tissue hypercalcemia, IL-17 mediated inflammation and apoptosis lead to cardiomyopathy: ultrastructural and biochemical findings. Toxicology 406–407:44–57. https://doi.org/10.1016/j-tox-2018-05-012
Yuan J, Li Q, Niu R, Wang J (2019) Fluoride exposure decreased learning ability and the expressions of the insulin receptor in male mouse hippocampus and olfactory bulb. Chemosphere 224:71–76. https://doi.org/10.1016/j-chemosphere-2019-02-113
Zhang J, Li Z, Qie M, Zheng R, Shetty J, Wang J (2016) Sodium fluoride and sulfur dioxide affected male reproduction by disturbing blood-testis barrier in mice. Food Chem Toxicol 94:103–111. https://doi.org/10.1016/j.fct.2016.05.017
Dhar V, Bhatnagar M (2009) Physiology and toxicity of fluoride. Indian J Dent Res 20(3):350–355. https://doi.org/10.4103/0970-9290-57379
Everett ET (2011) Fluoride’s effects on the formation of teeth and bones, and the influence of genetics. J Dent Res 90:552–560. https://doi.org/10.1177/00220-34510-384626
Qiao L, Liu X, He Y, Zhang J, Huang H, Bian W, Chilufya MM, Zhao Y, Han J (2021) Progress of signaling pathways, stress pathways and epigenetics in the pathogenesis of skeletal fluorosis. Int J Mol Sci 22(21):11932. https://doi.org/10.3390/ijms222111932
Chu Y, Gao Y, Yang Y, Liu Y, Guo N, Wang L, Huang W, Wu L, Sun D, Gu W (2020) β-catenin mediates fluoride-induced aberrant osteoblasts activity and osteogenesis. Environ Pollut 265(Pt A):114734. https://doi.org/10.1016/j-envpol-2020-114734
Xu H, Zhou YL, Zhang XY, Lu P, Li GS (2010) Activation of PERK signaling through fluoride-mediated endoplasmic reticulum stress in OS732 cells. Toxicology 277(1–3):1–5. https://doi.org/10.1016/j-tox-2010-08-006
Gu X, Han D, Chen W, Zhang L, Lin Q, Gao J, Fanning S, Han B (2016) SIRT1-mediated FoxOs pathways protect against apoptosis by promoting autophagy in osteoblast-like MC3T3-E1 cells exposed to sodium fluoride. Oncotarget 7(40):65218–65230. https://doi.org/10.18632/oncotarget-11573
Wang J, Zhao Y, Cheng X, Li Y, Xu H, Manthari RK, Wang J (2018) Effects of different Ca2+ level on fluoride-induced apoptosis pathway of endoplasmic reticulum in the rabbit osteoblast in vitro. Food Chem Toxicol 116(Pt B):189–195. https://doi.org/10.1016/j-fct-2018-04-013
Yu H, Jiang N, Yu X, Zhao Z, Zhang X, Xu H (2018) The role of TGFβ receptor 1-smad3 signaling in regulating the osteoclastic mode affected by fluoride. Toxicology 393:73–82. https://doi.org/10.1016/j-tox-2017-11-009
Lv YG, Kang L, Wu G (2016) Fluorosis increases the risk of postmenopausal osteoporosis by stimulating interferon γ. Biochem Biophys Res Commun 479(2):372–379. https://doi.org/10.1016/j-bbrc-2016-09-083
Bonewald LF (2017) The role of the osteocyte in bone and nonbone disease. Endocrinol Metab Clin North Am 46(1):1–18. https://doi.org/10.1016/j-ecl-2016-09-003
Komori T (2013) Functions of the osteocyte network in the regulation of bone mass. Cell Tissue Res 352(2):191–198. https://doi.org/10.1007/s00441-012-1546-x
Ru JY, Wang YF (2020) Osteocyte apoptosis: the roles and key molecular mechanisms in resorption-related bone diseases. Cell Death Dis 11:846. https://doi.org/10.1038/s41419-020-03059-8
Huber C, Collishaw S, Mosley JR, Reeve J, Noble BS (2007) Selective estrogen receptor modulator inhibits osteocyte apoptosis during abrupt estrogen withdrawal: implications for bone quality maintenance. Calcif Tissue Int 81:139–144. https://doi.org/10.1007/s00223-007-9049-6
Storlino G, Colaianni G, Sanesi L, Lippo L, Brunetti G, Errede M, Colucci S, Passeri G, Grano M (2020) Irisin prevents disuse-induced osteocyte apoptosis. J Bone Miner Res 35:766–775. https://doi.org/10.1002/jbmr-3944
Farr JN, Xu M, Weivoda MM, Monroe DG, Fraser DG, Onken JL, Negley BA, Sfeir JG, Ogrodnik MB, Hachfeld CM, LeBrasseur NK, Drake MT, Pignolo RJ, Pirtskhalava T, Tchkonia T, Oursler MJ, Kirkland JL, Khosla S (2017) Targeting cellular senescence prevents age-related bone loss in mice. Nat Med 23(9):1072–1079. https://doi.org/10.1038/nm.4385
Zhang Y, Yan M, Niu W, Mao H, Yang P, Xu B, Sun Y (2022) Tricalcium phosphate particles promote pyroptotic death of calvaria osteocytes through the ROS/NLRP3/Caspase-1 signaling axis in amouse osteolysis model. Int Immunopharmacol 107:108699. https://doi.org/10.1016/j.intimp.2022.108699
Jiang N, Guo F, Xu W, Zhang Z, Jin H, Shi L, Zhang X, Gao J, Xu H (2020) Effect of fluoride on osteocyte-driven osteoclastic differentiation. Toxicology 436:152429. https://doi.org/10.1016/j-tox-2020-152429
Yang J, Zhu Y, Zhang D, Yan Z, Zhao Y, Manthari RK, Cheng X, Wang J, Wang J (2021) Effects of different doses of calcium on the mitochondrial apoptotic pathway and Rho/ROCK signaling pathway in the bone of fluorosis rats. Biol Trace Elem Res 199(5):1919–1928. https://doi.org/10.1007/s12011-020-02305-6
Cui Y, Song M, Xiao B, Liu M, Liu P, Han Y, Shao B, Li Y (2021) ROS-mediated mitophagy and apoptosis are involved in aluminum-induced femoral impairment in mice. Chem Biol Interact 349:109663. https://doi.org/10.1016/j.cbi.2021.109663
Yin F, Yan J, Zhao Y, Guo KJ, Zhang ZL, Li AP, Meng CY, Guo L (2019) Bone marrow mesenchymal stem cells repair Cr (VI)-injured kidney by regulating mitochondria-mediated apoptosis and mitophagy mediated via the MAPK signaling pathway. Ecotoxicol Environ Saf 176:234–241. https://doi.org/10.1016/j.ecoenv.2019.03.093
Avila-Rojas SH, Aparicio-Trejo OE, Sanchez-Guerra MA, Barbier OC (2022) Effects of fluoride exposure on mitochondrial function: energy metabolism, dynamics, biogenesis and mitophagy. Environ Toxicol Pharmacol 94:103916. https://doi.org/10.1016/j.etap.2022.103916
Shen Y, Wu L, Qin D, Xia Y, Zhou Z, Zhang X, Wu X (2018) Carbon black suppresses the osteogenesis of mesenchymal stem cells: the role of mitochondria. Part Fibre Toxicol 15(1):16. https://doi.org/10.1186/s12989-018-0253-5
Matsuda N, Sato S, Shiba K, Okatsu K, Saisho K, Gautier CA, Sou YS, Saiki S, Kawajiri S, Sato F, Kimura M, Komatsu M, Hattori N, Tanaka K (2010) PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol 189(2):211–221. https://doi.org/10.1083/jcb-200910140
Ivankovic D, Chau KY, Schapira AH, Gegg ME (2016) Mitochondrial and lysosomal biogenesis are activated following PINK1/parkin-mediated mitophagy. J Neurochem 136(2):388–402. https://doi.org/10.1111/jnc-13412
Wang X, Ma H, Sun J, Zheng T, Zhao P, Li H, Yang M (2022) Mitochondrial ferritin deficiency promotes osteoblastic ferroptosis via mitophagy in type 2 diabetic osteoporosis. Biol Trace Elem Res 200(1):298–307. https://doi.org/10.1007/s12011-021-02627-z
Yang CN, Kok SH, Wang HW, Chang JZ, Lai EH, Shun CT, Yang H, Chen MH, Hong CY, Lin SK (2019) Simvastatin alleviates bone resorption in apical periodontitis possibly by inhibition of mitophagy-related osteoblast apoptosis. Int Endod J 52(5):676–688. https://doi.org/10.1111/iej-13055
Xu K, Lu C, Ren X, Wang J, Xu P, Zhang Y (2021) Overexpression of HIF-1α enhances the protective effect of mitophagy on steroid-induced osteocytes apoptosis. Environ Toxicol 36(11):2123–2137. https://doi.org/10.1002/tox-23327
Zhang Y, Yan M, Shan W, Zhang T, Shen Y, Zhu R, Fang J, Mao H (2022) Bisphenol A induces pyroptotic cell death via ROS/NLRP3/Caspase-1 pathway in osteocytes MLO-Y4. Food Chem Toxicol. https://doi.org/10.1016/j-fct-2021-112772
Bratic I, Trifunovic A (2010) Mitochondrial energy metabolism and ageing. Biochim Biophys Acta 1797(6–7):961–967. https://doi.org/10.1016/j.bbabio.2010.01.004
Zhang T, Shen Y, Zhu R, Shan W, Li Y, Yan M, Zhang Y (2022) Benzo[a]pyrene exposure promotes RIP1-mediated necroptotic death of osteocytes and the JNK/IL-18 pathway activation via generation of reactive oxygen species. Toxicology 476:153244. https://doi.org/10.1016/j-tox-2022-153244
Yan X, Wang L, Yang X, Qiu Y, Tian X, Lv Y, Tian F, Song G, Wang T (2017) Fluoride induces apoptosis in H9c2 cardiomyocytes via the mitochondrial pathway. Chemosphere 182:159–165. https://doi.org/10.1016/j-chemosphere-2017-05-002
Lv S, Zhang Y, Yan M, Mao H, Pan C, Gan M, Fan J, Wang G (2016) Inhibition of osteolysis after local administration of osthole in a TCP particles-induced osteolysis model. Int Orthop 40(7):1545–1552. https://doi.org/10.1007/s00264-015-3021-2
Yin J, Ni B, Liao WG, Gao YQ (2018) Hypoxia-induced apoptosis of mouse spermatocytes is mediated by HIF-1α through a death receptor pathway and a mitochondrial pathway. J Cell Physiol 233(2):1146–1155. https://doi.org/10.1002/jcp.25974
Wang X, Ni H, Xu W, Wu B, Xie T, Zhang C, Cheng J, Li Z, Tao L, Zhang Y (2021) Difenoconazole induces oxidative DNA damage and mitochondria mediated apoptosis in SH-SY5Y cells. Chemosphere 283:131160. https://doi.org/10.1016/j-chemosphere-2021-131160
Li Z, Guo D, Yin X, Ding S, Shen M, Zhang R, Wang Y, Xu R (2020) Zinc oxide nanoparticles induce human multiple myeloma cell death via reactive oxygen species and Cyt-C/Apaf-1/caspase-9/caspase-3 signaling pathway in vitro. Biomed Pharmacother 122:109712. https://doi.org/10.1016/j-biopha-2019-109712
Zhao Y, Wang J, Zhang J, Sun Z, Niu R, Manthari RK, Ommati MM, Wang S, Wang J (2022) Fluoride exposure induces mitochondrial damage and mitophagy via activation of the IL-17A pathway in hepatocytes. Sci Total Environ 804:150184. https://doi.org/10.1016/j.scitotenv.2021.15018
Fina BL, Lombarte M, Rigalli JP, Rigalli A (2014) Fluoride increases superoxide production and impairs the respiratory chain in ROS 17/2.8 osteoblastic cells. PLoS One 9(6):e100768. https://doi.org/10.1371/journal-pone-0100768
Suzuki M, Bandoski C, Bartlett JD (2015) Fluoride induces oxidative damage and SIRT1/autophagy through ROS-mediated JNK signaling. Free Radic Biol Med 89:369–378. https://doi.org/10.1016/j.freeradbiomed.2015.08.015
Wang S, Deng Z, Ma Y, Jin J, Qi F, Li S, Liu C, Lyu FJ, Zheng Q (2020) The role of autophagy and mitophagy in bone metabolic disorders. Int J Biol Sci 16(14):2675–2691. https://doi.org/10.7150/ijbs-46627
Gu X, Wang Z, Gao J, Han D, Zhang L, Chen P, Luo G, Han B (2019) SIRT1 suppresses p53-dependent apoptosis by modulation of p21 in osteoblast-like MC3T3-E1 cells exposed to fluoride. Toxicol In Vitro 57:28–38. https://doi.org/10.1016/j-tiv-2019-02-006
Li R, Gong Z, Yu Y, Niu R, Bian S, Sun Z (2022) Alleviative effects of exercise on bone remodeling in fluorosis mice. Biol Trace Elem Res 200(3):1248–1261. https://doi.org/10.1007/s12011-021-02741-y
Funding
This work was supported by the Natural Science Foundation of Zhejiang Province (LY21H060001), the National Natural Science Foundation of China (30900301), and the National Undergraduate Innovation and Entrepreneurship Training Program (202110349006).
Author information
Authors and Affiliations
Contributions
Yun Zhang conceived and designed the experiments. Fanhe Dong, Zihan Wang, Bingbing Xu, and Qiqi Wang carried out the experiments. Tao Zhang and Qiao Lin processed the data. Yun Zhang and Fanhe Dong wrote the paper. The final manuscript was read and approved by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Y., Dong, F., Wang, Z. et al. Fluoride Exposure Provokes Mitochondria-Mediated Apoptosis and Increases Mitophagy in Osteocytes via Increasing ROS Production. Biol Trace Elem Res 201, 3994–4007 (2023). https://doi.org/10.1007/s12011-022-03450-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03450-w