Abstract
Atrial fibrillation is the most frequently encountered arrhythmia following cardiac surgery. Since the essential trace elements zinc, copper, and magnesium are suspected to have an effect on postoperative atrial fibrillation, the concentrations of these elements were determined by flame atomic absorption spectrophotometry in the plasma of 60 patients undergoing elective coronary artery bypass grafting. Blood samples were collected every 30 min during cardiopulmonary bypass and postoperatively. Plasma concentrations of copper, zinc, and magnesium were measured with flame atomic absorption spectrophotometry. All patients were monitored by continuous electrocardiography until they became outpatients or immediately after atrial fibrillation had taken place. Atrial fibrillation occurred in 13 of the 60 patients, corresponding to 21.7%. The zinc and copper concentrations at postoperative days 1 and 3 were significantly different (P < 0.05) between patients with and without atrial fibrillation. The concentrations of zinc following cardiopulmonary bypass recovered more slowly in patients with postoperative atrial fibrillation than in patients without it. Whether or not supplemental zinc could lower the incidence of postoperative atrial fibrillation should be evaluated in future prospective randomized clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atrial fibrillation (AF) is the most frequently encountered arrhythmia following cardiac surgery, although its incidence varies widely in reported studies, commonly ranging from 20% to 70% [1–3]. The risk of postoperative atrial fibrillation (POAF) increases with advancing age and the complexity of the surgical procedure [2, 4, 5]. Although POAF is usually a well-tolerated problem, it does, in many cases, require specific therapy, together with the length of hospital stay, more healthcare costs, and it may even be associated with increased mortality [5–8].
The pathophysiology of POAF is complex and its etiology multifactorial. Recent studies have implicated inflammation as a potential contributing factor in the development of POAF, and elevated levels of C-reactive protein and pro-inflammatory cytokines have been associated with its occurrence [9–11].
Twenty-seven randomized multiple studies and meta-analyses [12, 13] have demonstrated that compared with placebo, β-blocker therapy helps to reduce the risk of AF by up to 61%. As a result, routine administration of β-blockers after coronary artery bypass graft (CABG) has become the standard of medical care at many institutions, and ours is no exception. Unfortunately, β-blockade alone is still associated with a 20–30% incidence of postoperative atrial arrhythmias [12, 13]; therefore, further improvements are needed.
During cardiac surgery with cardiopulmonary bypass (CPB), the possible occurrence of a series of immunological and inflammatory alterations may trigger oxidative stress [14, 15]. Under the nonphysiological conditions during the CPB, the alterations related to ischemia–reperfusion bring about a large amount of free radicals [16], which might be responsible for systemic inflammation and considerable structural and functional cellular lesion [17].
Trace elements (TE; e.g., Zn, Cu, and Mg) are micronutrients predominantly obtained from their dietary intake. Zn is a functional component of enzymes that helps to protect biomembranes against peroxidative damage [18, 19]. It presents in many enzymes of the biomembranes and is necessary for maintaining the structure of the cellular membrane. Also, it has a decisive role in several biological processes, including protein synthesis, nucleic acid, carbohydrate, and lipid metabolism. In some of its physiological functions, zinc resembles an antioxidant as it protects membranes against lipoperoxidation, and proteins against denaturation [18, 19]. Moreover, as an essential component of the dismutase superoxide enzyme, it inactivates the superoxide radicals and turns them into less harmful forms. A major role of zinc in relation to the cardiac cells is to decrease the formation of hydroxyl radicals, which are known to be highly injurious to the myocardium [20]. Additionally, Zn deficiency may increase the susceptibility of the phospholipid cell membrane to free radical damage and oxidative changes [21].
The role of Cu is still under discussion, however. TE such as Cu, cobalt, and arsenic may contribute to myocardial dysfunction [22, 23]. In addition, copper-deficient diets produce anemia, decrease liver Zn/Cu–superoxide dismutase (SOD), and cardiac cytochrome C oxidase activity [24]. On the other hand, in the body, Zn and Cu levels are regulated and interact with each another. Therefore, variations in the Zn content and the Zn/Cu ratio reflect the effects of these microelements [25].
Mg is an important determinant of the resting membrane potential of cardiac cell membranes. It regulates the sodium–potassium–adenosine triphosphatase pump, thus affecting the intracellular/extracellular potassium ratio and the membrane resting potential of myocardial cells [26]. Extracellular magnesium deficiency results in the loss of cellular potassium and gain of cellular sodium, which leads to an increase in myocardial excitability [27]. Mg inhibits the influx of calcium through sarcolemmal channels and modulates cyclic adenosine monophosphate, thus blocking the slow inward calcium current, whereas hypomagnesemia results in the increase of intracellular calcium. A recent systematic review of the observed effects of intravenous Mg2+ after CABG surgery concluded that Mg2+ prevents POAF [28].
There are some studies evaluating trace elements in patients submitted to operations with cardiopulmonary bypass. However, as none of these trials focused on the relationships between TE and POAF, we monitored the changes of the plasma Zn, Cu, and Mg levels in patients with POAF that submitted to coronary artery bridge grafts with cardiopulmonary bypass and looked for possible associations between them.
Methods
Patient Population
After approval of ethics committee, 60 consecutive patients presenting for elective initial CABG on CPB were recruited to participate in this study. Written and informed consent was obtained from each patient. All patients have had β-blocker therapy for more than 1 week. Those undergoing concurrent valvular surgery and CABG or repeated surgery were excluded from the study as were the patients with a preoperative history of paroxysmal or persistent AF, renal insufficiency (serum creatinine greater than 110 μmol/L), asthma, and chronic obstructive pulmonary disease. Clinical information was collected from all patients by reviewing their medical records.
Operative Technique
By using the same CPB protocol, all patients were assigned with antegrade cold blood cardioplegia with Mg (5 g/L). Cold blood cardioplegia was retrograded after completion of each distal anastomosis. Cold blood cardioplegia or cold blood was retrograded during each proximal anastomosis. Cold blood cardioplegia was antegraded after completion of each proximal anastomosis for all patients with CABG. Ice was used for topical cooling. Body temperature was diminished from 32°C to 34°C depending on the anticipated time length of CPB. The patients were subjected to moderate hemodilution at a flow rate of 2 L min−1 m−2, and the mean systemic pressure was maintained between 50 and 80 mmHg. At 30 min of CPB, one dose of magnesium sulfate (25%, 10 mL) was injected into CPB circuit. After the operation, the patients were transferred into the intensive care unit equipped with an auxiliary breathing machine. The patients were allowed to take food, six hours after they were disengaged from the ventilator. On the first and the second day after the operation, magnesium sulfate, 25% 10 mL, was supplied with other liquids. In addition, operative variables such as the cross-clamp time, the CPB time, and the postoperative time on the auxiliary breathing machine were recorded for comparison.
Blood Sampling and Analysis
Blood samples for the trace element determinations were collected after the induction of anesthesia and before CPB (T1), at 30 min (T2), 60 min (T3), and 90 min (T4) of bypass (unless the CPB ceased), 8 h after the CPB ceased (T5), 1 day (T6), 3 days (T7), and 6 days (T8) postoperatively.
All samples (3-mL blood) were collected in tubes containing a standard amount of lithium heparin. The sample was centrifuged at 3,000 rpm for 5 min, and the plasma was separated and preserved in decontaminated containers. Plasma Zn, Cu, and Mg determinations were made with flame atomic absorption spectrophotometry, the process of which was completed in Tianjin Kingmed Center for Clinic Laboratory. Samples were stored at 4°C in well-decontaminated containers if it happened that they could not be analyzed immediately. A BH5100 multicenter atomic absorption spectrophotometer (Beijing Bohui Innovation Technology Co., Ltd) was used for instrumental analyses. λmax 213.9, 324.7, and 285.2 nm were used for Zn, Cu, and Mg level assessment, respectively. Subsequently, the concentrations were determined following the preparation of calibration curves and evaluation of line equations [26, 28].
Inspection of the Changes of Rhythm
All patients were monitored by continuous electrocardiography until they became outpatients or immediately after POAF had taken place.
Statistical Analysis
The statistical analyses were performed with SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). The data were expressed as the means ± SEM and were analyzed applying the independent t test. The analysis of variance test was used in the comparisons of the quantitative variables at each time point for the patients in each group. The independent t test was used for comparing the quantitative variables among patients in each group at every moment. Values of p less than 0.05 were considered significant.
Results
Patient Characteristics
Baseline and operative characteristics of patients are given in Table 1. The occurrence of POAF had been recorded in 13 out of 60 patients (21.7%), 2, 4, 4, 1, 1, and 1 case at the first, second, third, fourth, fifth, and eighth day after the operation, respectively. There is no significant difference in age, stature, weight, CPB duration, cross clamping, and breathing machine auxiliary postoperation in two groups (Table 1). None of the patients developed heart failure or other serious complications in the early postoperative period.
Plasma Zn Changes Before and Following CPB
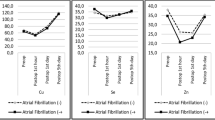
The plasma Zn concentrations changed in each group during the study period; these changes were statistically significant with p < 0.01. Table 2 and Fig. 1 depict the alterations of the Zn concentrations. It decreased after the onset of CPB and reached the lowest concentration at T4 and then gradually recovered after the CPB ceased. The concentration of Zn was completely recovered at the postoperative day 6 (T8). By comparison, the Zn concentration was significantly higher in CABG patients without POAF than in patients with POAF (p < 0.05) at T6 and T7 (Table 2 and Fig. 1).
Changes of zinc concentrations during and after cardiopulmonary bypass in patients undergoing coronary artery bypass grafting (CABG). **P < 0.01 compared to T1. # P < 0.05 compared between CABG with POAF and without POAF. Blood samples were collected after the induction of anesthesia and before CPB (T1), at 30 min (T2), 60 min (T3), and 90 min (T4) of bypass (unless the CPB ceased), 8 h after the CPB ceased (T5). The samples were also taken at the postoperative days 1 (T6), 3 (T7), and 6 (T8)
Plasma Cu Changes Before and Following CPB
The Cu concentration was altered during the study period (p < 0.01) in each group. Table 2 and Fig. 2 depict the alterations of Cu concentrations. It decreased after the onset of CPB and reached the lowest concentration at T4 and then gradually recovered after the CPB was ceased. The concentration of Cu was completely recovered at the postoperative day 3 (T7) and was even higher at the day 6 (T8) than the preoperative value. By comparison, the Cu concentration was significantly higher in CABG patients with POAF than in patients without POAF (p < 0.05) at T6 and T7 (Table 2 and Fig. 2).
Changes of copper concentrations during and after cardiopulmonary bypass in patients undergoing coronary artery bypass grafting (CABG). **P < 0.01 compared to T1. # P < 0.05 compared between CABG with POAF and without POAF. See Fig. 1 for the meaning of T1–T8
Plasma Mg Changes Before and Following CPB
What is more, the Mg concentration was also altered during the study period (p < 0.01) in each group. However, opposite to the concentrations of Zn and Cu that decreased after the onset of CPB, the Mg concentration increased after the onset of CPB and reached the highest concentration at T2 and then gradually recovered after that time. Obviously, our CPB protocol includes the addition of Mg (1 g/L) in the blood cardioplegia as well as the addition of 2.5 g Mg (magnesium sulfate) into the CPB circuit at 30 min of CPB (see “Methods” section) that should be responsible for the increase of the Mg concentration after the onset of CPB. The concentration of Mg was completely recovered at postoperative day 6 (T8). Table 2 and Fig. 3 depict the alterations of the Mg concentrations. By comparison, there were no significant differences regarding the Mg concentration between the two groups (p > 0.05; Table 2 and Fig. 3).
Changes of magnesium concentrations during and after cardiopulmonary bypass in patients undergoing coronary artery bypass grafting (CABG). **P < 0.01 compared to T1. # P < 0.05 compared between CABG with POAF and without POAF. See Fig. 1 for the meaning of T1–T8
Discussion
The results of this study show that (1) the concentrations of Zn and Cu decreased considerably during cardiopulmonary bypass, (2) the concentrations of Zn and Cu recovered gradually after cardiopulmonary bypass, arriving at preoperation levels at the sixth day postoperation, (3) the recovery of Zn and Cu concentrations after CPB demonstrates the differences between patients with POAF and without POAF, and (4) with the current protocol of CPB, the concentration of Mg is sufficiently maintained.
Studies evaluated the changes of plasma or serum TE, such as Zn, Cu, and Mg, in patients submitted to operations with cardiopulmonary bypass. The concentrations of TE in plasma decreased considerably during cardiopulmonary bypass. Variability in blood levels could be partly accounted for hemodilution caused by the administration of colloids and crystalloids without TE. It has been demonstrated that Cu and Zn blood concentrations were considerably lower in patients who develop heart failure in the early postperfusion period than in those with an uncomplicated postoperative period [29]. Zn, Cu, and Mg concentrations increased during postoperation period [30]. However, little is known about the relation between the concentration of TE and POAF.
Zn has been shown to elicit mitogenic actions in many cellular populations [30, 31]. Thus, Zn levels were found to be higher in peri-infarct tissue, and notably in the microsomal fraction of myocardial cells, with an attendant increase of the activities of Zn-dependent enzymes, reflecting the ongoing repair of cell damage [32]. In patients with advanced clinical heart failure, the observation of profound hypozincemia was much clearer [33].
Cu also plays an important part in preserving the mitochondria1 function of cells in skeletal and cardiac muscle. A decrease in the tissue level of this cation can influence the late recovery after a myocardial ischemic attack [34]. The decreased Zn levels have been associated with deterioration in Cu levels and function [35]. Cu and Zn have both been stated to have an important role in protecting membrane structures from toxic injuries produced by superoxide anions. This action is mediated by enzymatic activities such as that of superoxide dismutase, which can regulate the membrane lipid peroxidation [36, 37].
In the body, Zn and Cu levels affect one another [24]. Since both Zn and Cu are crucial in the regulation of antioxidant enzymes such as Cu/Zn-SOD [38], a significant imbalance between Cu and Zn blood concentrations may contribute to a higher systemic oxidative stress. Irrespective of the etiology, oxidative stress is important and correlates with the severity of symptoms and signs of heart failure [39, 40]. These defenses (such as Cu/Zn-SOD) could be overwhelmed, thus creating an antioxidant deficit particularly in cases when the activity of these oxireductases is dependent on Zn concentrations. This deficit may be further worsened if Zn bioavailability is compromised [41]. Zn deficiency due to malnutrition was also associated with a decline in Cu/Zn-SOD activity [41].
The present study demonstrates that with current CPB protocols, the maintenance of Mg is adequate. In fact, we did not find any Mg deficiency in our patients. This is obviously due to the active supplement of Mg during and after CPB. But we have not gained a lower incidence of POAF, like in another study [42]. And we find that Zn concentration recovered more slowly after operation in patients undergoing CABG with POAF. Moreover, copper concentration changes reversely. It is observed at first to third day after operation, when it is much easier for POAF to take place.
In conclusion, our study has demonstrated that the current CPB protocol is adequate for the maintenance of Mg levels during CPB. However, the low Zn and Cu concentrations observed in the present study may suggest that, in the future, consideration will have to be given to the need of supplementing Zn and Cu in cardiac surgery. Whether or not supplemental zinc could lower the incidence of postoperative atrial fibrillation should be evaluated in future prospective randomized clinical trials.
References
Lauer MS, Eagle KA, Buckley MJ et al (1989) Atrial fibrillation following coronary artery bypass surgery. Prog Cardiovasc Dis 31:367–378
Mathew JP, Fontes ML, Tudor IC et al (2004) A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA 291:1720–1729
Kaireviciute D, Aidietis A, Lip GY (2009) Atrial fibrillation following cardiac surgery: clinical features and preventative strategies. Eur Heart J 30:410–425
Creswell LL, Schuessler RB, Rosenbloom M et al (1993) Hazards of postoperative atrial arrhythmias. Ann Thorac Surg 56:539–549
Auer J, Weber T, Berent R et al (2005) Postoperative atrial fibrillation independently predicts prolongation of hospital stay after cardiac surgery. J Cardiovasc Surg (Torino) 46:583–588
Bramer S, Straten AH, Hamad MS et al (2010) The impact of new-onset postoperative atrial fibrillation on mortality after coronary artery bypass grafting. Ann Thorac Surg 90:443–450
Aranki SF, Shaw DP, Adams DH et al (1996) Predictorsof atrial fibrillation after coronary artery surgery. Current trends and impact on hospital resources. Circulation 94:390–397
Villareal RP, Hariharan R, Liu BC et al (2004) Postoperative atrial fibrillation and mortality after coronary artery bypass surgery. J Am Coll Cardiol 43:742–748
Ishii Y, Schuessler RB, Gaynor SL et al (2005) Inflammation of atrium after cardiac surgery is associated with inhomogeneity of atrial conduction and atrial fibrillation. Circulation 111:2881–2888
Tselentakis EV, Woodford E, Chandy J et al (2006) Inflammation effects on the electrical properties of atrial tissue and inducibility of postoperative atrial fibrillation. J Surg Res 135:68–75
Ishida K, Kimura F, Imamaki M et al (2006) Relation of inflammatory cytokines to atrial fibrillation after off-pump coronary artery bypass grafting. Eur J Cardiothorac Surg 29:501–505
Connolly S, Cybulsky I, Lamy A et al (2003) Double-blind, placebo-controlled, randomized trial of prophylactic metoprolol for reduction of hospital length of stay after heart surgery: the beta-Blocker Length of Stay (BLOS) study. Am Heart J 145:226–232
Crystal E, Connolly SJ, Sleik K et al (2002) Interventions on prevention of postoperative atrial fibrillation in patients undergoing heart surgery: a meta-analysis. Circulation 106:75–80
Clermont G, Vergely C, Jazayeri S et al (2002) Systemic free radical activation is a major event involved in myocardial oxidative stress related to cardiopulmonary bypass. Anesthesiology 96:80–87
Christen S, Finckh B, Lykkesfeld J et al (2005) Oxidative stress precedes peak systemic inflammatory response in pediatric patients undergoing cardiopulmonary bypass operation. Free Radic Biol Med 38:1323–1332
Bolli R, Jeroudi MO, Patel BS et al (1989) Marked reduction of free radical generation and contractile dysfunction by antioxidant therapy begun at the time of reperfusion: evidence that myocardial “stunning” is a manifestation of reperfusion injury. Circ Res 65:607–622
Valko M, Morris H, Cronin MT (2005) Metals toxicity and oxidative stress. Curr Med Chem 12:1161–1208
Bray TM, Bettger WJ (1990) The physiological role of Zn as an antioxidant. Free Radic Biol Med 8:281–291
Powell SR (2000) The antioxidant properties of Zn. J Nutr 130(Suppl 5S):S1447–S1454
Hennig B, Toborek M, Mcclain CJ (1996) Antiatherogenic properties of Zn: implications in endothelial cell metabolism. Nutrition 12:711–717
de Lorgeril M, Salen P, Accominotti M et al (2001) Dietary and blood antioxidants in patients with chronic heart failure. Insights into the potential importance of selenium in heart failure. Eur J Heart Fail 3:661–669
Shokrzadeh M, Ghaemian A, Salehifar E et al (2009) Serum Zn and copper levels in ischemic cardiomyopathy. Biol Trace Elem Res 127:116–123
Medeiros DM, Liao Z, Hamlin RL (1992) Electrocardiographic activity and cardiac function in copper-restricted rats. Proc Soc Exp Biol Med 200:78–84
Tang YR, Zhang SQ, Xiong Y et al (2003) Studies of five microelement contents in human serum, hair, and fingernails correlated with aged hypertension and coronary heart disease. Biol Trace Elem Res 92:97–104
Elin RJ (1994) Magnesium: the fifth but forgotten electrolyte. Am J Clin Pathol 102:616–622
Altura BM, Altura BT (1996) Role of magnesium in pathophysiological processes and the clinical utility of magnesium ion selective electrodes. Scand J Clin Lab Invest Suppl 56:211–234
Dementéva II, Andrianova MIu, Dzemeshkevich SL et al (1993) Changes in the content of microelements—copper, Zn and iron—in the blood of patients following cardiopulmonary bypass. Anesteziol Reanimatol 4:50–53
Shepherd J, Jones J, Frampton GK et al (2008) Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation. Health Technol Assess 12:iii–v, ix-95
Nuutinen LS, Ryhänen P, Pihlajaniemi R et al (1981) The levels of Zn, copper, calcium and magnesium in serum and urine after heart-valve replacement. Effects of oxygenator type and postoperative parenteral nutrition. Infusionsther Klin Ernahr 8:214–217
Chvapil M (1976) Effect of Zn on cells and biomembranes. Med Clin North Am 60:799–812
Riordan JF (1976) Biochemistry of Zn. Med Clin North Am 60:661–674
Zamparelli R, Carelli G, Pennisi MA et al (1986) Zn and copper metabolism during open-heart surgery. Scand J Thorac Cardiovasc Surg 20:241–245
Ghaemian A, Salehifar E, Jalalian R et al (2011) Zn and copper levels in severe heart failure and the effects of atrial fibrillation on the Zn and copper status. Biol Trace Elem Res 143:1239–1246
Chipperfield B, Chipperfield JR (1978) Differences in metal content of the heart muscle in death from ischemic heart disease. Am Heart J 95:732–737
Milne DB, Davis CD, Nielsen FH (2001) Low dietary Zn alters indices of copper function and status in postmenopausal women. Nutrition 17:701–708
Chvapil M, Ryan JN, Zukoski CF (1972) Effects of Zn on lipid peroxidation in liver microsomes and mitochondria. Proc Soc Exp Biol Med 141:150–153
O’Dell BL (1976) Biochemistry of copper. Med Clin North Am 60:687–703
Cousins RJ (1985) Absorption, transport, and hepatic metabolism of copper and Zn: special reference to metallothionein and ceruloplasmin. Physiol Rev 65:238–309
Ungvári Z, Gupte SA, Recchia FA et al (2005) Role of oxidative-nitrosative stress and downstream pathways in various forms of cardiomyopathy and heart failure. Curr Vasc Pharmacol 3:221–229
Wykretowicz A, Furmaniuk J, Smielecki J et al (2004) The oxygen stress index and levels of circulating interleukin-10 and interleukin-6 in patients with chronic heart failure. Int J Cardiol 94:283–287
Thakur S, Gupta N, Kakkar P (2004) Serum copper and Zn concentrations and their relation to superoxide dismutase in severe malnutrition. Eur J Pediatr 163:742–744
Cook RC, Humphries KH, Gin K et al (2009) Prophylactic intravenous magnesium sulphate in addition to oral {beta}-blockade does not prevent atrial arrhythmias after coronary artery or valvular heart surgery: a randomized, controlled trial. Circulation 15:s163–s169
Acknowledgments
The assistance of the surgical team and nurses in the Cardiac Operating Theater, TEDA International Cardiovascular Hospital is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yan, YQ., Zou, LJ. Relation Between Zinc, Copper, and Magnesium Concentrations Following Cardiopulmonary Bypass and Postoperative Atrial Fibrillation in Patients Undergoing Coronary Artery Bypass Grafting. Biol Trace Elem Res 148, 148–153 (2012). https://doi.org/10.1007/s12011-012-9356-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-012-9356-2