Abstract
The proper concentrations of plasma bioelements may favorably reduce the incidence of metabolic disorders, which often occur during immunosuppressive therapy. Mycophenolate mofetil (MMF) is currently one of the most frequently administered immunosuppressive agents; however, MMF treatment is often related to gastrointestinal side effects. The aim of this study was thus to verify whether the MMF treatment itself, or its metabolite pharmacokinetics, has an effect on the concentrations of plasma bioelements. To determine this, the effect of MMF on the levels of both major (sodium [Na], potassium [K], calcium [Ca], magnesium [Mg]), and trace (iron [Fe], zinc [Zn], copper [Cu]) plasma bioelements in 61 renal transplant recipients was assessed in comparison to a control group (n = 45). The pharmacokinetic parameters of mycophenolic acid were determined by the high-performance liquid chromatography method. All patients filled out a 24-h diet history questionnaire. The results showed high plasma concentrations of Fe and low plasma concentrations of Mg and Zn as compared with diagnostic norms. The patients treated with MMF had significantly lower plasma Na (P < 0.001) and significantly higher plasma Zn (P = 0.030) and Cu concentrations (P < 0.001). In conclusion, MMF treatment was found to affect plasma Fe, Zn, and Cu levels by increasing their concentrations while decreasing the plasma Na concentration. Mg and Zn deficiencies, as well as excessive Fe levels, are frequently observed irrespective of the immunosuppressive regimen applied, which suggests that monitoring of these bioelements may be favorable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Successful renal transplantation is chiefly achieved by suppression of the process of transplant rejection. In clinical transplantology, different immunosuppressive regimens are applied, among which corticosteroids and calcineurin inhibitors (CNI) (cyclosporine [CsA] and tacrolimus [Tac]), as well as mycophenolate mofetil (MMF), are the most common agents [1]. Secondly, it is known that adequate nutritional status may improve the post-transplant outcomes, as malnutrition is one of the main complications in the late post-transplant period. Proper concentrations of plasma bioelements may favorably reduce the incidence of metabolic disorders, which frequently occur during immunosuppressive therapy [2].
Following oral administration, pharmacologically inactive MMF is rapidly and entirely hydrolyzed into an active metabolite, mycophenolic acid (MPA). MPA is a non-nucleoside, uncompetitive, reversible inosine monophosphate dehydrogenase inhibitor which prevents acute rejection of the transplanted organ and also decreases the frequency of late rejection after 1 year and consecutive years [3, 4].
It has been shown that MMF administration is not associated with increases in lipid levels, neurotoxicity, or nephrotoxicity, and neither does it affect blood pressure. In addition, MMF administration allows reduction of CsA and Tac doses both of which demonstrate nephrotoxicity [5, 6]. However, MMF therapy is often associated with gastrointestinal side effects such as diarrhea, nausea, vomiting, abdominal pain, and gastrointestinal bleeding [7]. Over 20% of patients treated with MMF suffer from anemia [8, 9]. Therapies consisting of CsA and Tac administration may also be accompanied by diarrhea and vomiting [10], which decreases the bioavailability of nutritional components such as the mineral elements.
So far, there have been some studies describing the influence of CNI on the concentration of some mineral elements; however, little research has been done to evaluate the influence of MMF on the concentrations of major (calcium [Ca], magnesium [Mg], potassium [K], sodium [Na]), and trace (copper [Cu], iron [Fe], zinc [Zn]) plasma bioelements. As MMF is known for frequent gastrointestinal disorders, the aim of this study was to determine whether MMF treatment or MPA pharmacokinetics influences plasma bioelements in renal transplant recipients.
Materials and Methods
Study Population
This study considered 106 renal transplant patients aged 20–72 years (mean, 46 ± 13 years), treated with different immunosuppressive regimens. All patients were in the late post-transplant period (>6 months) and the transplants were performed in the period 1996–2008. The characteristics of the patients are presented in Table 1.
Of the 106 renal transplant recipients, 61 patients (aged 23–69 years; mean, 43 ± 12 years) were included in the study group as they had been treated with MMF-based immunosuppressive regimens. MMF was administered in combination with CsA (n = 28) or Tac (n = 24), or without CNI (n = 9). Almost all patients in the study group were treated with corticosteroids (88.5%).
The control group consisted of 45 renal transplant recipients aged 20–72 years who had been treated with various immunosuppressive regimens excluding MMF and the sodium salt of MPA. Corticosteroids (n = 42) and CNI (n = 36) were the most frequently administered, and azathioprine (n = 16) and sirolimus (n = 12) were also applied.
The study was approved by the Bioethical Committee at Poznan University of Medical Sciences and is in accordance with the Helsinki Declaration of 1975. Informed consent was obtained from the patients prior to initiating the study.
Assessment of Plasma Bioelements
Plasma bioelements levels (Fe, Mg, Zn, and Cu) were determined in 106 patients from both the study and control groups. For all patients, the data on K, Na, and Ca concentrations were obtained from hospital registers. The determination of these bioelements is routinely performed in the hospital laboratory at each of the patient’s transplant clinic appointments.
For the plasma bioelement analyses, the patients’ blood samples were taken after an overnight fast and collected in ethylenediaminetetraacetic acid (EDTA) tubes, subsequently centrifuged to obtain plasma, and kept at −20°C until analyzed.
The plasma concentrations of Fe, Zn, Cu, and Mg were determined by flame atomic absorption spectrometry (AAS-3, Carl Zeiss, Germany). In order to obtain concentrations of the plasma bioelements which were within the ranges of the calibration curves, the samples were diluted as follows: for Fe, Zn, and Cu analyses, 0.01% aqueous solution of Triton X-100 (Merck) was used, while for the Mg analysis, aqueous solutions consisting of 0.01% Triton X-100 (Merck) and 0.05% lanthanum chloride (Merck) were used.
For Fe and Zn analyses, the calibration curves were performed within the 0.5–2.5 ppm range (8.9–44.7 and 7.6–38.2 μmol/L for Fe and Zn, respectively), while for Mg and Cu analyses, the range was within 0.25–2.00 ppm (0.01–0.08 mmol/L and 3.9–31.4 μmol/L for Mg and Cu, respectively). The precision of the method was determined with certified Human Multi-Sera Level 2 (Randox) and amounted to 95.6%, 105.7%, 104.4%, and 99.6% for Fe, Zn, Cu, and Mg, respectively.
Assessment of Dietary Intake
Each patient was given a diet history questionnaire to fill out in order to estimate the 24-h food intake on the day preceding the collection of the blood samples. On the basis of the patients’ dietary history, the intake of the major and trace elements was analyzed with Microsoft Access 2000 databases containing the extended version of the “Food composition tables” [11].
Information on the supplementation of bioelements was obtained from the diet history questionnaire as well as from the patients’ hospital registers. For this purpose, a period of 1 month directly before the examination was taken into consideration or in cases where the supplementation was longer than 3 months, the 6 months preceding the examination were considered.
Assessment of MPA Pharmacokinetic Profiles
The 4-h MPA pharmacokinetic profiles were determined in the study group (n = 61). Firstly, the blood samples were collected before the morning dose of MMF (time 0), and subsequently at 40 min, 1 h, 2, 3, and 4 h after MMF oral administration. Blood samples were collected in EDTA tubes, centrifuged to obtain plasma, and kept at −20°C until analyzed.
The pharmacokinetic profiles of MPA were determined according to the high-performance liquid chromatography method described elsewhere [12].
The following MPA pharmacokinetic parameters were calculated: predose concentration (C 0), maximum concentration (C max), and area under the concentration–time curve from 0 to 4 h (AUC0–4 h), using the linear trapezoidal rule.
Definitions of Normal Levels
The reference norms of the plasma mineral elements for adult patients were assumed to be 137–145 mmol/L for Na, 3.6–5.0 mmol/L for K, 2.10–2.55 mmol/L for Ca (norms according to which supplementation was adjusted, established in the laboratory of the District Hospital in Poznań), 0.70–1.05 mmol/L for Mg, 10.6–28.3 μmol/L for Fe, 10.1–16.8 μmol/L for Zn, and 11–24.4 μmol/L for Cu [13, 14].
For K and Na, the minimum dietary intake levels were 3,500 and 575 mg/day, respectively. Recommended dietary intakes of 900 mg/day for Ca, 280–350 mg/day for Mg, 15–18 mg/day for Fe, 13–16 mg/day for Zn, and 2.0–2.5 mg/day for Cu were assumed, following recommended dietary allowances [15].
Statistical Analysis
All statistical tests were performed using Statistica software version 8.0 (StatSoft), and P values lower than 0.05 were considered significant. Normality was determined by the Shapiro–Wilk test. The differences between variables were estimated using the Mann–Whitney test and for normally distributed data, Student’s t test was applied. The results are presented as means ± standard deviations. The correlations of the data were tested using Pearson or Spearman correlation analysis for normally and non-normally distributed data, respectively. Pearson’s chi-square test was used for the evaluation of qualitative data.
Multivariate analysis of variance (MANOVA) and multivariate regression analysis were applied to evaluate the interactions between sex, age, post-transplant period, MMF treatment, pharmacokinetics of MMF metabolite, drugs co-administered (CNI and diuretics), and their influence on the concentrations of plasma bioelements studied. The nonsignificant models are not presented.
Results
Baseline Characteristics
The patients from the study and control groups did not differ statistically in body weight, creatinine clearance, creatinine concentration, co-administered drugs (corticosteroids, angiotensin-converting enzyme inhibitors [ACEi], angiotensin II receptor blockers, diuretics such as loop and thiazide diuretics or thiazide analogs), or in bioelement supplementation.
The patients from the study group who were treated with MMF and CsA did not differ in sex, age, period after kidney transplantation, creatinine clearance, or creatinine concentration from those patients treated with MMF and Tac (data not shown). The MMF mean dose was lower (1.06 ± 0.27 g/day) when co-administered with Tac as compared with the MMF mean dose co-administered with CsA (1.73 ± 0.35 g/day). Gastrointestinal disorders were more frequently observed in study group (73.9% vs. 48.9%; P = 0.003).
Levels of Plasma Bioelements
In patients treated with MMF, significantly higher plasma Fe, Zn, and Cu concentrations, as well as significantly lower plasma Na concentrations were found relative to the control group (Table 2).
In the study group, both hypernatremia (4.6% vs. 20.0%; P = 0.002) and Cu concentrations below the norm (6.9% vs. 40.0%; P < 0.001) were less frequently observed than in the control group.
Within the study group, in the subgroup of patients treated with MMF and CsA, hypomagnesemia was less frequently observed (44.0% vs. 87.0%; P = 0.003), concurrent with significantly higher plasma concentrations of Mg (P = 0.004) and Cu (P = 0.037) as compared to the subgroup of patients treated with MMF and Tac (Table 2).
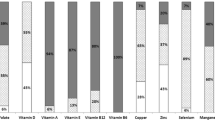
Considering all patients, plasma concentrations of Fe exceeded the normal levels for this component in most patients (59%). Mg and Zn plasma concentrations were below the norms in 53% and 90% of the patients, respectively (Fig. 1).
Bioelements and MPA Pharmacokinetic Parameters
There were significant positive correlations between the MPA pharmacokinetic parameters (AUC0–4 h and C 0) and the Mg concentrations, as well as negative correlations between the MPA pharmacokinetic parameters (AUC0–4 h, C 0 and C max) and the Na and K concentrations (Table 3).
Patients with Mg concentrations within the norm presented higher values of MPA pharmacokinetic parameters such as MPA AUC0–4 h (32.77 ± 12.37 vs. 22.70 ± 7.91 mg h/L; P = 0.002), MPA C 0 (3.89 ± 2.49 vs. 2.35 ± 1.58 mg/L; P = 0.005) and MPA C max (16.49 ± 6.18 vs. 11.53 ± 4.92 mg/L; P = 0.004) as compared to the patients with hypomagnesemia.
Multivariate Regression Analysis
In multivariate regression analysis, the influence of MMF treatment and its pharmacokinetic parameters in combination with some clinical factors (diuretics and CNI co-administered, sex, and age) on plasma bioelement concentrations were evaluated. There was a significant influence of MPA AUC0–4 h and co-administered diuretics on K concentrations (P < 0.001; Table 4). However, despite the great statistical significance of this model, the partial P value for MPA AUC0–4 h was statistically insignificant in this model. Additionally, MMF administration and diuretic treatment influenced Na concentration. In the studied regression models, neither did ACEi treatment influence K concentrations, nor did diuretic treatment influence Ca and Mg concentrations. Mg concentration was significantly dependent on MPA C 0 and on CNI applied (P = 0.011), while Zn concentration was dependent on MMF treatment, sex, and age (P = 0.003). However, partial P values for MPA C 0 in Mg model and for MMF treatment in Zn model were statistically insignificant. For Fe, Cu, and Ca, no statistically significant models were found in the multivariate regression analysis.
Multivariate Analysis of Variance
On account of the differences in age, sex, and post-transplant period between patients treated with MMF and the control group (Table 1), the influence of these variables on plasma bioelement concentrations was assessed. In univariate analysis, higher Zn concentrations were observed in men (8.42 ± 2.14 vs. 7.50 ± 1.68 μmol/L; P = 0.042), in younger patients (8.57 ± 1.99 vs. 7.19 ± 1.53 μmol/L; P < 0.001), as well as in patients with shorter post-transplant periods (<5 years) in comparison to patients with longer post-transplant periods (>5 years; 8.26 ± 1.99 vs. 7.34 ± 1.84 μmol/L; P = 0.015). However, despite the differences in age, sex, and post-transplant period between the study and control groups, the MANOVA for Zn did not show any interactions between MMF treatment and the factors described. While in the univariate analysis, patients younger than 50 years presented statistically lower Na concentrations (141.8 ± 3.0 vs. 143.6 ± 2.6 mmol/L; P = 0.007), the MANOVA did not show any interactions between MMF treatment and the age of patients for either Na or Zn. No influence of gender, age, or post-transplant period on the concentrations of Fe, Mg, Ca, Cu, or K was observed.
Dietary Intake and Supplementation
Current dietary intakes of the bioelements in daily food ration were also analyzed in the patients included in the study (Table 5). The dietary supplies of Ca, Mg, Fe, Zn, and Cu were lower than recommended. Concomitantly, similar intakes of the analyzed bioelements were observed in the study group as compared to the control group as well as within the study group with respect to CNI applied (P > 0.05). Nevertheless, mean Na intakes in all patients included in the study exceeded the reference ranges by a factor of four.
Discussion
In order to assess the impact of MMF on nutritional status in renal transplant recipients, the concentrations of major and trace elements in the study and control groups were compared. Subsequently, the influence of MMF metabolites on plasma bioelement levels was analyzed.
The influence of MMF on plasma Na presented in our study may be favorable, as Na intake in examined patients exceeded the reference ranges fourfold, despite the fact that Na restriction is normally recommended to renal transplant recipients. Higher plasma Zn and lower plasma Na levels in patients treated with MMF may have been related to the patients’ younger ages and, in the case of Zn, additionally to the shorter post-transplant period in the study group, as compared to the control group. However, the multivariate analysis of variance excluded the influence of the factors described on these results. Additionally, the dietary intakes of the analyzed bioelements were similar in both studied groups, and no pharmacological supplementation of these elements was provided. The results obtained thus indicate that MMF may be the factor affecting Na and Zn balances. The relation observed between MMF and plasma Na concentration may partially confirm the results of Mattson et al. [16] who observed the attenuation of blood pressure elevation in sodium-sensitive rats treated with MMF and fed excessively with sodium chloride. No research concerning MMF influence on Cu and Zn plasma levels has been found in the literature and the explanation of the interaction mechanisms between MMF and plasma concentrations of the studied bioelements thus requires further research. However, the results suggest that MMF may also affect Cu balance, as higher concentrations—along with less frequent occurrence of Cu levels below the norm—were found in the study group, concomitantly with lower than recommended dietary supply in all patients.
Although the patients treated with MMF demonstrated similar plasma K as compared to the control group, there were slightly significant negative correlations between K levels and all MPA pharmacokinetic parameters, as well as the MMF dose. The correlation concerning K, as well as Na, may be related to the decreased absorption of these electrolytes in patients treated with MMF, probably due to gastrointestinal disorders which were more frequent in the study group. Plasma K is routinely monitored and supplemented in renal transplant patients, which may be the reason for the occurrence of similar K levels in both the study and control groups. In this case too, no similar studies concerning MMF have been found in the literature.
Our study did not show any differences in Ca concentrations between the study and control groups. We found only one article concerning Ca concentrations in patients treated with MMF. The authors observed decreased urinary excretion of Ca during MMF treatment, in combination with CsA and prednisone; however, the study involved a small number of patients (n = 20) and the results were compared to normal values [17]. As CsA is known to decrease Ca concentrations in patients’ serum by increasing the urinary excretion of this bioelement [18], the results of Khosroshahi et al. [17] suggest that MMF may reduce the Ca excretion.
Higher plasma Fe in the group treated with MMF along with Fe concentrations above the normal level, regardless of the immunosuppressive drug administered, may be due to the frequency of Fe supplementation (about 28% of study group vs. 18% of control group, P > 0.05). This supplementation is applied to prevent anemia, which is one of the most frequent side effects during immunosuppressive therapy [19]. The high plasma Fe in all patients included in our study was related neither to gender nor to the patients’ current dietary intake as we observed low and insufficient dietary supplies of Fe in their food rations (76.4% of Fe intake norm). The results indicate that the introduction of Fe monitoring may be favorable and may assist in rapid diet correction as well as in adjusting pharmacological supplementation.
Although we found some positive correlations between Mg plasma concentrations and MPA pharmacokinetic parameters, no similar studies concerning MMF and Mg have been found in the literature. Further research is thus required in order to explain the cause of the correlations obtained. Our study showed lower plasma levels of Mg in patients treated with MMF and Tac, as compared to patients treated with MMF and CsA. Numerous studies have shown that CNI leads to decreased Mg concentration [2, 20–26], mainly due to Mg loss with urine [21, 22]. In our study, the differences in plasma Mg between patients treated with MMF and CsA in comparison to MMF and Tac may be caused by the different daily doses of MMF applied in these two subgroups. Our results suggest that in patients treated with CNI, the introduction of MMF into immunosuppressive therapy may be beneficial by reducing the risk of hypomagnesemia as MMF correlates positively with Mg concentration.
In all patients included in the study, the deviations from the normal levels of plasma bioelements were observed. Insufficiency of plasma Zn and Mg, as well as too high concentrations of Fe—which is often excessively supplemented after kidney transplantation, were most frequently observed. Zn deficiency may have been caused by corticosteroid treatment (about 91% of patients) as corticosteroids increase the excretion of Zn, K, and Ca. Lower than normal Mg concentrations may have been the result of frequent (about 33%) diuretic co-administration as these drugs are known to increase Mg, K, and Na excretion. Importantly, neither Zn nor Mg was routinely monitored in patients included in the study in contrast to K, Na, and Ca.
In conclusion, in our study, MMF treatment favorably affected Cu and Zn levels by increasing their plasma concentrations and favorably affected Na levels by decreasing its plasma concentration. However, some of these differences, despite being statistically significant, may be irrelevant in clinical practice. Yet the results suggest that MMF does not limit the absorption of bioelements studied with the exception for Na. The concentrations of Na, K, and Ca are routinely determined in post-transplant patients. It is, however, reasonable to also monitor other plasma bioelements, such as Mg, Zn, and Fe, as the concentrations of these components diverged frequently from normal levels. This may enable early diet and supplement adjustment in renal transplant recipients.
References
Kaufman DB, Shapiro R, Lucey MR, Cherikh WS, Bustami TR, Dyke DB (2004) Immunosuppression: practice and trends. Am J Transplant 4:38–53
Teplan V, Valkovsky I, Teplan V, Stollova M, Vyhnanek F, Andel M (2009) Nutritional consequences of renal transplantation. J Renal Nutr 19:95–100
Mamelok R (2005) From mechanisms to long-term benefits. Transplantation 79:43–44, suppl 3
Arns W (2007) Noninfectious gastrointestinal (GI) complications of mycophenolic acid therapy: a consequence of local GI toxicity? Transplant Proc 39:88–93
Cox VC, Ensom MHH (2003) Mycophenolate mofetil for solid organ transplantation: does the evidence support the need for clinical pharmacokinetic monitoring? Ther Drug Monit 25:137–157
Staatz CE, Tett SE (2007) Clinical pharmacokinetics and pharmacodynamics of mycophenolate in solid organ transplant recipients. Clin Pharmacokinet 46:13–58
Moder KG (2003) Mycophenolate mofetil: new applications for this immunosuppressant. Ann Allergy Asthma Immunol 90:15–19
Behrend M (2001) Adverse gastrointestinal effects of mycophenolate mofetil: etiology, incidence and management. Drug Saf 24:645–663
Ott U, Busch M, Steiner T, Wolf G (2008) Anemia after renal transplantation: an underestimated problem. Transplant Proc 40:3481–3484
Webster A, Woodroffe RC, Taylor RS, Chapman JR, Craig JC (2005) Tacrolimus versus cyclosporine as primary immunosuppression for kidney transplant recipients. Cochrane Database Syst Rev 19:CD003961
Kunachowicz H, Nadolna I, Przygoda B, Iwanow K (eds) (2005) Food composition tables (in Polish) PZWL. Warsaw, Poland
Kamińska J, Głyda M, Sobiak J, Chrzanowska M (2011) Pharmacokinetics of mycophenolic acid and its phenyl glucuronide metabolite in kidney transplant recipients with renal impairment. Arch Med Sci (in press)
Hartwich J, Wybrańska I, Maziarz B (2002) Wartości referencyjne podstawowych wyników badań w diagnostyce laboratoryjnej. In: Dembińska-Kieć A, Naskalski JW (eds) Diagnostyka laboratoryjna z elementami biochemii klinicznej (in Polish). Elsevier, Wrocław, pp 933–959
Hartwich J, Zdzienicka A, Mazian B (2010) Wartości podstawowych wyników badań w diagnostyce laboratoryjnej. In: Dembińska-Kieć A, Naskalski JW (eds) Diagnostyka laboratoryjna z elementami biochemii klinicznej (in Polish). Elsevier, Wrocław, pp 1035–1056
Ziemlański Ś, Bułhak-Jachymczyk B, Budzyńska-Toporowska J, Panczenko-Kresowska B, Wartanowicz M (1998) Normy żywienia dla ludności w Polsce (energia, białko, tłuszcz, witaminy i składniki mineralne) (in Polish). Nowa Med 4:1–28
Mattson DL, James L, Berdan EA, Meister CJ (2006) Immune suppression attenuates hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension 48:149–156
Khosroshahi HT, Shoja MM, Azar SA, Tubbs RS, Safa J, Etemadi J, Ardalan MR (2007) Calcium and phosphorus metabolism in stable renal transplant recipients. Exp Clin Transplant 5:670–672
Zahmatkesh M, Kadkhodaee M, Ghaznavi R, Mahdavi-Mazdeh M (2007) Acid-base status determines cyclosporine-induced hypercalciuria. Transplant Proc 39:1231–1232
Lorenz M, Kletzmayr J, Huber A, Hörl WH, Sunder-Plassmann G, Födinger M (2005) Iron overload in kidney transplants: prospective analysis of biochemical and genetic markers. Kidney Int 67:691–697
Atsmon J, Dolev E (2005) Drug-induced hypomagnesaemia: scope and management. Drug Saf 28:763–788
Navaneethan SD, Sankarasubbaiyan S, Gross MD, Jeevanantham V, Monk RD (2006) Tacrolimus-associated hypomagnesemia in renal transplant recipients. Transplant Proc 38:1320–1322
Nijenhuis T, Hoenderop JG, Bindels RJ (2004) Downregulation of Ca(2+) and Mg(2+) transport proteins in the kidney explains tacrolimus (FK506)-induced hypercalciuria and hypomagnesemia. J Am Soc Nephrol 15:549–557
Lote CJ, Thewles A, Wood JA, Zafar T (2000) The hypomagnesaemic action of FK506: urinary excretion of magnesium and calcium and the role of parathyroid hormone. Clin Sci 99:285–292
Ahmadi F, Naseri R, Lessan-Pezeshki M (2009) Relation of magnesium level to cyclosporine and metabolic complications in renal transplant recipients. Saudi J Kidney Dis Transpl 20:766–769
Aisa Y, Mori T, Nakazato T, Shimizu T, Yamazaki R, Ikeda Y, Okamoto S (2005) Effects of immunosuppressive agents on magnesium metabolism early after allogeneic hematopoietic stem cell transplantation. Transplantation 80:1046–1050
Arthur JM, Shamim S (2000) Interaction of cyclosporine and FK506 with diuretics in transplant patients. Kidney Int 58:325–330
Acknowledgments
This study was supported by grant no. 501-02-03306413-02324-50382 from Poznan University of Medical Sciences.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Kamińska, J., Sobiak, J., Suliburska, J.M. et al. Effect of Mycophenolate Mofetil on Plasma Bioelements in Renal Transplant Recipients. Biol Trace Elem Res 145, 136–143 (2012). https://doi.org/10.1007/s12011-011-9178-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-011-9178-7