Abstract
Although maternal, fetal, and placental mechanisms compensate for disturbances in the fetal environment, any nutritional inadequacies present during pregnancy may affect fetal metabolism, and their consequences may appear in later life. The aim of the present study is to investigate the influence of maternal diet during gestation on Fe, Zn, and Cu levels in the livers and kidneys of adult rats. The study was carried out on the offspring (n = 48) of mothers fed either a protein-balanced or a protein-restricted diet (18% vs. 9% casein) during pregnancy, with or without folic acid supplementation (0.005- vs. 0.002-g folic acid/kg diet). At 10 weeks of age, the offspring of each maternal group were randomly assigned to groups fed either the AIN-93G diet or a high-fat diet for 6 weeks, until the end of the experiment. The levels of Fe, Zn, and Cu in the livers and kidneys were determined by the F-AAS method. It was found that postnatal exposure to the high-fat diet was associated with increased hepatic Fe levels (p < 0.001), and with decreased liver Zn and Cu contents (p < 0.01 and p < 0.05, respectively), as well as with decreased renal Cu contents (p < 0.001). Moreover, the offspring’s tissue mineral levels were also affected by protein and folic acid content in the maternal diet. Both prenatal protein restriction and folic acid supplementation increased the liver Zn content (p < 0.05) and the kidney Zn content (p < 0.001; p < 0.05, respectively), while folic acid supplementation resulted in a reduction in renal Cu level (p < 0.05). Summarizing, the results of this study show that maternal dietary folic acid and protein intake during pregnancy, as well as the type of postweaning diet, affect Fe, Zn, and Cu levels in the offspring of the rat. However, the mechanisms responsible for this phenomenon are unclear, and warrant further investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The great impact of maternal nutrition during pregnancy on the progeny’s growth and development is well-known. Through numerous epidemiological and molecular biology studies, much evidence has been accumulated to support the hypothesis on the fetal origins of adult diseases [1]. Developmental plasticity enables the metabolism of a fetus to be adjusted to the nutritional environment in utero. However, the consequences of these adaptations are persistent, and may be responsible for increased susceptibilities to diet-related diseases later in life [2]. It has been postulated that this adverse effect of fetal programming is a consequence of a mismatch between prenatal and postnatal nutrition [3].
Several groups of nutrients have been considered as programming factors, protein and micronutrients among them [4]. It has been shown that protein and folic acid levels in the maternal diet can determine the lipid and carbohydrate metabolism of the progeny, and interaction effects between prenatal and postnatal nutritional environments have been observed [5–9]. Moreover, it has been suggested that folic acid supplementation may reverse the long-term effects caused by protein deficiency during pregnancy [10, 11]. In animal models, the effect of various micronutrient restrictions on the outcome pregnancy has also been examined [12–14]. The results observed were dependent on the type of micronutrient that was restricted and on the gender of the progeny. The most commonly observed result of maternal micronutrient deficiencies was growth retardation in the progeny. Moreover, in later development, the effect of fetal micronutrient malnutrition manifested as changes in body composition, in insulin sensitivity, and in lipid metabolism [15]. Additionally, protein deficiency and folic acid supplementation per se may affect mineral status; however, the direction of such changes and the mechanisms involved are still unclear [16–19].
It has been hypothesized that the adaptive changes in the progeny’s metabolism induced by maternal protein deficiency or by folic acid supplementation could also influence mineral homeostasis, including Fe, Zn, and Cu homeostasis, which might be manifested as changes in the tissue content of these minerals in the offspring. Moreover, Fe, Zn, and Cu act as cofactors for many enzymatic reactions in numerous metabolic pathways. Hence, metabolism and accumulation of minerals determined by maternal nutrition can affect the overall metabolism and contribute to the phenomenon of fetal programming. The objective of this study was thus to investigate the influence of the maternal diet during gestation on tissue Fe, Zn, and Cu levels in the progeny. To assess the interaction effect between prenatal and postnatal nutrition, the experimental model also included high-fat feeding after weaning. In this model, postnatal high-fat feeding was introduced to exacerbate metabolic changes caused by prenatal exposition to protein deficiency. Additionally, there are some data suggesting that protein restriction during gestation promotes a preference for high-fat foods in the young adult offspring rats, especially in females [5].
Material and Methods
Animals, Diets, and Study Design
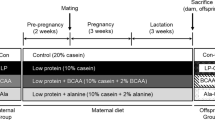
The experiment was performed with the agreement of the local bioethics committee (approval no. 35/2007). Rats were housed in individual cages on a 12-h light/12-h dark cycle, at a temperature of 20°C. Twenty virgin female Wistar rats (five per group) were mated with eight male rats. After confirming mating by observing the appearance of vaginal plugs on the cage floor, dams were assigned to one of the four groups fed isocaloric diets: the NP-NF group, fed a normal protein, normal folic acid (FA) diet (18% protein, 0.002-g FA per kilogram diet); the PR-NF group, fed a protein-restricted, normal folic acid diet (9% protein, 0.002-g FA per kilogram diet); the PR-FS group, fed a protein-restricted, folic acid-supplemented diet (9% protein, 0.005 g FA per kilogram); and the NP-FS group, fed a normal protein, folic acid-supplemented diet (18% protein, 0.005 g FA per kilogram). Each diet was supplemented with l-cystine, with the protein-restricted diets being supplemented with a quantity of l-cystine proportional to the protein content. The full composition of the diets is presented in Table 1. To minimize variation in nutrition during the suckling period, litters were culled to a maximum of eight pups within 3 days of delivery.
At 10 weeks of age, the offspring of each mother group were randomly assigned to two groups that were fed either a high-carbohydrate, low-fat AIN-93G diet (AIN groups), or a low-carbohydrate, high-fat diet (HF groups) until the age of 16 weeks (postweaning period). Thus, eight groups of six animals each (three males, three females) were created. The high-fat diet contained 39.5% fat by weight, provided by sunflower oil and lard (Table 1). The total dietary energy was determined by bomb calorimetry, using a KL-11 Mikado calorimeter (Precyzja-BIT, Bydgoszcz, Poland).
Food intake was measured daily during pregnancy, as well as throughout the postweaning period. The weight gain of the mothers and the offspring was measured weekly. Daily intakes of Fe, Zn, and Cu were calculated as the average daily intake of each microelement per 100 g of body weight. At the end of the experimental period, the animals were anesthetized with sodium thiopental injection (40 mg/kg body weight) and killed by cardiac puncture. The liver and kidneys were dissected, accurately weighted, and kept frozen (−70°C) until analysis for Fe, Zn, and Cu contents.
Microelement Determination
Tissue samples (∼1 g) were digested in 65% (w/w) spectra pure HNO3 (Merck) in the Microwave Digestion System (MARS 5, CEM Corp., USA). Thereafter, the concentrations of Fe, Zn, and Cu in the mineral solutions were measured using flame atomic absorption spectrometry method (AAS-3, Carl Zeiss, Jena, Germany).
The accuracy of the quantitative mineral determinations was assured by simultaneous analysis of the certified reference material (pig kidney BCR® no. 186, Brussels), that reached for Fe, Zn, and Cu: 92%, 93%, and 98%, respectively.
Statistical Analysis
The results are presented in Tables 2, 3, and 4 as group means with standard errors. The statistical analysis was carried out using STATISTICA 8 for Windows. The effect of maternal diet, offspring sex, and postweaning diets on the analyzed data was assessed using a multiway ANOVA, followed by a post hoc Scheffé’s test. P < 0.05 was accepted as statistically significant.
Results
The average intakes of dietary micronutrients (Fe, Zn, and Cu) were significantly different between the groups. Since the diet consumption was lower in the HF groups than in the AIN groups, the average daily intake of Fe, Zn, and Cu was about 27% lower in rats fed the high-fat diet (Table 2).
Postnatal high-fat feeding changed the mineral homeostasis in adult rats in comparison to rats fed the standard laboratory chow. The main determinants of the hepatic Fe stores were sex (p < 0.001) and fat content in the postnatal diet (p < 0.001) (Tables 3 and 4). Despite the lower daily microelement intake in the high-fat fed rats, hepatic Fe levels increased by 34% in these animals. Higher hepatic levels of Fe were also found in female rats fed the high-fat diet postnatally (p < 0.05). Neither prenatal exposure to protein deficiency nor folic acid supplementation alone influenced this parameter. However, an interaction effect between these two factors was found (p < 0.01). The highest hepatic Fe levels were observed in PR-FS group, but prenatal nutrition did not affect renal Fe content. High-fat feeding was associated with the higher level of Fe in kidneys (not statistically significant). The interaction effects were detected between protein content in the maternal diet, fat content in the postweaning diet, and sex, and also between folic acid content in the maternal diet, fat content in the postweaning diet, and sex (p < 0.05 and p < 0.01, respectively).
Postnatal high-fat feeding resulted in the adult rats’ hepatic Zn content being decreased by 8% (p < 0.01), but this was less marked in the female rats (Tables 3 and 4). It can partly be explained by the lower postnatal intake of Zn in the HF groups. Maternal protein deficiency, as well as folic acid supplementation, increased the hepatic Zn content (by 7% and 9%, respectively) in the adult offspring (p < 0.05). There was an interaction effect between prenatal maternal folic acid supplementation and postnatal high-fat feeding (p < 0.05), and the highest Zn stores were observed in the progeny of folic acid-supplemented dams fed the low-fat diet postnatally. Postnatal exposure to the high-fat diet did not significantly affect renal Zn contents. Similar to the results concerning liver Zn content, the low protein and high folic acid contents in the maternal diet were associated with increased renal Zn levels, at p < 0.001 and p < 0.05, respectively. There was also an interaction between these two factors (p < 0.05). The highest level of Zn was detected in the liver of the PR-FS rats (128.7 ± 3.1 μg/g d.m.) in contrast to all the other groups, where Zn content was about 110 μg/g d.m.
In the rats fed the high-fat diet, hepatic Cu contents were decreased by 20% (p < 0.05), independently of prenatal nutrition, and renal Cu levels declined by 25% (p < 0.001). It can be a result of lower intake of Cu with the postnatal diet. Interestingly, the decreased renal Cu content was also associated with maternal folic acid supplementation (p < 0.05). Moreover, in the progeny of dams of the PR-FS group, the kidney Cu levels were much lower than in the other maternal groups (NP-NF, 46.4 ± 4.1 μg/g d.m.; PR-NF, 50.1 ± 7.2 μg/g d.m.; PR-FS, 35.6 ± 3.3 μg/g d.m.; NP-FS, 45.3 ± 7.0 μg/g d.m.). There was also an interaction effect between protein and folic acid content in the maternal diet and fat content in the postweaning diet (p < 0.05). The lowest level of Cu in kidneys was observed in the PR-FS HF group (34.0 ± 4.7 μg/g d.m.), while the highest level was observed in the PR-NF AIN group (63.5 ± 12.3 μg/g d.m.). Female progeny even increased their accumulation of renal Cu to 90.7 ± 4.5 μg/g d.m.
Discussion
Maternal protein restriction during pregnancy has been reported to induce long-term changes in the metabolism of progeny [1]. The programming effect of the maternal diet may differ depending on the sex of the progeny [9, 20, 21]. Moreover, the mineral content of tissue changes over the lifespan and differences between the sexes in microelement accumulation may arise in cases of Fe, Zn, and Cu [22]. In this study, sex was a determinant of mineral content in the rat liver and kidneys; specifically, female rats had higher hepatic and renal levels of these minerals than males. However, to analyze the main influence of sex and also its interaction effects (along with other experimental factors), further studies are needed making use of larger groups of animals.
The accumulation of microelements is also regulated by the ongoing food intake. In this study, it has been shown that postnatal high-fat feeding affects mineral status in young adult rats. Independent of maternal dietary history, consumption of the high-fat diet resulted in the increased hepatic Fe content. A similar effect was observed in the case of the renal Fe stores. Factors that can affect Fe metabolism have been intensively investigated in recent years, especially with respect to dietary fat. It was shown that the higher the fat level is in the diet, the greater the Fe absorption, independent of the Fe form [23]; our study confirms this observation. Despite the almost 30% lower overall microelement intake in the high-fat fed rats, the hepatic Fe stores of these animals were about 25% higher than in the animals fed the low-fat diet. Higher Fe accumulation may account for higher risk of type 2 diabetes [24]. It has been shown that elevated iron stores are associated with increased oxidative stress and insulin resistance, which can be induced by high-fat feeding [25–27]. Iron overload is often associated with decreased insulin sensitivity [28, 29]. In the study of Wapnir and Devas [30], rats fed a 45% fat diet had increased hepatic Fe content. On the other hand, Guenno et al. [31] found that the splenic Fe content decreased, but plasma transferrin concentration increased in the Wistar rats fed a high-fat diet (45% of energy) for 6 weeks.
The impact of dietary fat on tissue Zn and Cu stores has been poorly investigated so far. In this study, the diet rich in saturated fats decreased the hepatic and the renal Cu levels, as well as the hepatic Zn stores in the rats, irrespective of maternal dietary history. Our results are in agreement with those obtained by Jalili et al. [32], who investigated whether a high-fat diet with a 2:1 saturated–polyunsaturated fatty acid ratio exacerbates signs of Cu deficiency. It was found that the consumption of such a high-fat diet caused a decrease in the hepatic Cu content, and that this effect was more pronounced when a Cu deficiency already existed. The mineral status is regulated at various points, with intestinal absorption being one of them. It has been demonstrated that dietary free fatty acids and triglycerides inhibit Cu absorption in the small intestine of rats, probably due to the formation of poorly soluble Cu soaps [33]. Therefore, the decreased Cu accumulation observed in the present study may be a result of impaired Cu absorption. The level of accumulated Zn and Cu can also be affected by the accumulation of other minerals. The interactions between Fe and other elements (e.g., Zn and Cu) are well-known [34, 35]. It was found that increased absorption of Fe after feeding high-fat diets rich in saturated fats may also affect the bioavailability of Cu [36].
Although in the literature there is data suggesting that folic acid may affect zinc absorption, this interaction seems to be very subtle [16–19]. In this study, the offspring of mothers fed the protein-deficient, folic acid-supplemented diet had increased hepatic Zn contents. The interaction between folate intake and heme at the level of intestinal absorption has been recently reported. A proton-coupled folate transporter also acts as a heme transporter protein [37]. Our results, however, did not reflect such a relationship, and folic acid supplementation of the maternal diet did not affect tissue Fe levels in the progeny.
Recent studies have shown that the intake of nutrients involved in DNA methylation—such as methionine, choline, and B vitamins—can affect this process. Moreover, maternal intake of these nutrients can permanently change methylation patterns in the progeny [1]. Therefore, it might be assumed that changes in DNA methylation status may affect the storage of minerals, as was observed in this study. To our knowledge, the impact of folic acid and protein content in the maternal diet on micronutrient metabolism has not yet been analyzed. However, a very recent study showed that protein restriction alters the hepatic expression of genes involved in ion transport, developmental processes, and response to reactive oxygen species. Folic acid supplementation prevents these changes in the response to reactive oxygen species pathways, but not in ion transport or developmental processes [11].
In conclusion, the results of this study have shown that high-fat feeding can influence micronutrient status. However, micronutrient metabolism can also be determined by protein and folic acid content in the maternal diet, but a comprehensive explanation of the mechanisms responsible for this effect requires further research.
References
Chmurzynska A (2010) Fetal programming: link between early nutrition, DNA methylation, and complex diseases. Nutr Rev 68(2):87–98
Stocker CJ, Cawthorne MA (2008) The influence of leptin on early life programming of obesity. Trends Biotechnol 26(10):545–551
Gluckman PD, Hanson MA (2008) Developmental and epigenetic pathways to obesity: an evolutionary-developmental perspective. Int J Obes (Lond) 32(Suppl 7):S62–S71
McArdle HJ, Andersen HS, Jones H et al (2006) Fetal programming: causes and consequences as revealed by studies of dietary manipulation in rats—a review. Placenta 27(Suppl A):S56–S60
Bellinger L, Lilley C, Langley-Evans SC (2004) Prenatal exposure to a maternal low-protein diet programmes a preference for high-fat foods in the young adult rat. Br J Nutr 92(3):513–520
Burdge GC, Phillips ES, Dunn RL et al (2004) Effect of reduced maternal protein consumption during pregnancy in the rat on plasma lipid concentrations and expression of peroxisomal proliferator-activated receptors in the liver and adipose tissue of the offspring. Nutr Res 24:639–646
Erhuma A, Salter AM, Sculley DV et al (2007a) Prenatal exposure to a low-protein diet programs disordered regulation of lipid metabolism in the aging rat. Am J Physiol Endocrinol Metab 292(6):E1702–E1714
Erhuma A, Bellinger L, Langley-Evans SC et al (2007b) Prenatal exposure to undernutrition and programming of responses to high-fat feeding in the rat. Br J Nutr 98(3):517–524
Burdge GC, Lillycrop KA, Jackson AA et al (2008) The nature of the growth pattern and of the metabolic response to fasting in the rat are dependent upon the dietary protein and folic acid intakes of their pregnant dams and post-weaning fat consumption. Br J Nutr 99(3):540–549
Lillycrop KA, Phillips ES, Jackson AA et al (2005) Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr 135(6):1382–1386
Lillycrop KA, Rodford J, Garratt ES et al (2010) Maternal protein restriction with or without folic acid supplementation during pregnancy alters the hepatic transcriptome in adult male rats. Br J Nutr 103(12):1711–1719
Padmavathi IJ, Kishore YD, Venu L et al (2009) Prenatal and perinatal zinc restriction: effects on body composition, glucose tolerance and insulin response in rat offspring. Exp Physiol 94(6):761–769
Venu L, Kishore YD, Raghunath M (2005) Maternal and perinatal magnesium restriction predisposes rat pups to insulin resistance and glucose intolerance. J Nutr 135(6):1353–1358
Venu L, Padmavathi IJ, Kishore YD et al (2008) Long-term effects of maternal magnesium restriction on adiposity and insulin resistance in rat pups. Obesity 16(6):1270–1276
Christian P, Stewart CP (2010) Maternal micronutrient deficiency, fetal development, and the risk of chronic disease. J Nutr 140(3):437–445
Milne DB, Canfield WK, Mahalko JR et al (1984) Effect of oral folic acid supplements on zinc, copper, and iron absorption and excretion. Am J Clin Nutr 39(4):535–539
Kauwell GP, Bailey LB, Gregory JF 3rd et al (1995) Zinc status is not adversely affected by folic acid supplementation and zinc intake does not impair folate utilization in human subjects. J Nutr 125(1):66–72
Green TJ, Skeaff CM, Whiting SJ et al (2003) Effect of folic acid supplementation on plasma zinc concentrations of young women. Nutrition 19(6):522–523
Tupe RS, Chiplonkar SA, Agte VV (2007) Changes in zinc uptake in response to ascorbic acid and folic acid in rat liver slices under normal and oxidative stress conditions. Biofactors 30(1):27–34
Bellinger L, Langley-Evans SC (2005) Fetal programming of appetite by exposure to a maternal low-protein diet in the rat. Clin Sci (Lond) 109(4):413–420
Gallou-Kabani C, Gabory A, Tost J et al (2010) Sex- and diet-specific changes of imprinted gene expression and DNA methylation in mouse placenta under a high-fat diet. PLoS ONE 21;5(12):e14398
Uchino E, Tsuzuki T, Inoue K (1990) The effects of age and sex on seven elements of Sprague–Dawley rat organs. Lab Anim 24(3):253–264
Johnson PE, Lukaski HC, Bowman TD (1987) Effects of level and saturation of fat and iron level and type in the diet on iron absorption and utilization by the rat. J Nutr 117:501–507
Schümann K (2001) Safety aspects of iron in food. Ann Nutr Metab 45(3):91–101
Buettner R, Parhofer KG, Woenckhaus M et al (2006) Defining high-fat-diet rat models: metabolic and molecular effects of different fat types. J Mol Endocrinol 36(3):485–501
Iwashita S, Tanida M, Suzuki M (2002) Decreased skeletal muscle insulin receptors in high-fat diet-related hypertensive rats. Nutr Res 22(9):1049–1053
Kim J-Y, Nolte LA, Hansen PA et al (2000) High-fat diet-induced muscle insulin resistance: relationship to visceral fat mass. Am J Physiol Regul Integr Comp Physiol 279:R2057–R2065
Cooksey RC, Jones D, Gabrielsen S et al (2010) Dietary iron restriction or iron chelation protects from diabetes and loss of beta-cell function in the obese (ob/ob lep−/−) mouse. Am J Physiol Endocrinol Metab 298(6):E1236–E1243
Hatunic M, Finucane FM, Brennan AM et al (2010) Effect of iron overload on glucose metabolism in patients with hereditary hemochromatosis. Metabolism 59(3):380–384
Wapnir RA, Devas G (1995) Copper deficiency: interaction with high-fructose and high-fat diets in rats. Am J Clin Nutr 61:105–110
Le Guenno G, Chanséaume E, Ruivard M et al (2007) Study of iron metabolism disturbances in an animal model of insulin resistance. Diabetes Res Clin Pract 77(3):363–370
Jalili T, Medeiros DM, Wildman RE (1996) Aspects of cardiomyopathy are exacerbated by elevated dietary fat in copper-restricted rats. J Nutr 126(4):807–816
Wapnir RA, Sia MC (1996) Copper intestinal absorption in the rat: effect of free fatty acids and triglycerides. Proc Soc Exp Biol Med 211(4):381–386
Cohen NL, Keen CL, Lönnerdal B et al (1985) Effects of varying dietary iron on the expression of copper deficiency in the growing rat: anemia, ferroxidase I and II, tissue trace elements, ascorbic acid, and xanthine dehydrogenase. J Nutr 115(5):633–649
Rossander-Hultén L, Brune M, Sandström B et al (1991) Competitive inhibition of iron absorption by manganese and zinc in humans. Am J Clin Nutr 54(1):152–156
Ebesh O, Barone A, Harper RG et al (1999) Combined effect of high-fat diet and copper deficiency during gestation on fetal copper status in the rat. Biol Trace Elem Res 67(2):139–150
Laftah AH, Latunde-Dada GO, Fakih S et al (2009) Haem and folate transport by proton-coupled folate transporter/haem carrier protein 1 (SLC46A1). Br J Nutr 101(8):1150–1156
Acknowledgments
This work was supported in part by the Ministry of Science and Higher Education, grant no. N N312 151034.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Król, E., Krejpcio, Z. & Chmurzynska, A. Folic Acid and Protein Content in Maternal Diet and Postnatal High-Fat Feeding Affect the Tissue Levels of Iron, Zinc, and Copper in the Rat. Biol Trace Elem Res 144, 885–893 (2011). https://doi.org/10.1007/s12011-011-9048-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-011-9048-3