Abstract
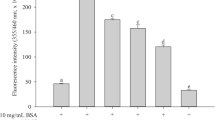
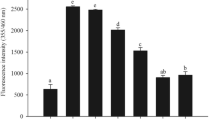
Nonenzymatic glycation of long-lived proteins has been implicated in several complications related to age and diabetes. Dicarbonyl compounds such as methylglyoxal (MGO) have been identified as the predominant source for the formation of advanced glycation end-products (AGEs) in various tissues. We investigated the effect of 13 micronutrients on MGO-mediated in vitro glycation of bovine serum albumin (BSA), as formation of AGEs and protein carbonyls. BSA (10 mg/ml) was incubated at 37°C with 100 mM MGO for 24 hours, in presence of ascorbic acid, Trolox (water-soluble α-tocopherol analog), β-carotene, retinol, riboflavin, thiamin, folic acid, niacin, pyridoxine, zinc, iron, manganese, and selenium. Fluorescence was measured at the wavelength pair of 370 and 440 nm as an index of the formation of AGEs and spectra were recorded for promising interactions at λex = 280 nm and λex = 370 nm. Within four standard antiglycating agents, aminoguanidine showed highest inhibitory response for BSA glycation followed by quercetin, gallic acid, and tannic acid. Promising antiglycation potential was seen for Trolox, riboflavin, Zn, and Mn as evidenced by decrease in the formation of AGEs and protein carbonyls.






Similar content being viewed by others
References
Eble A, Thorpe S, Baynes J (1983) Nonenzymatic glycation and glucose-dependent cross linking of protein. J Biol Chem 258:9406–9412
Chellan P, Nagaraj R (1999) Protein crosslinking by the Maillard reaction: dicarbonyl-derived imidazolium crosslinks in aging and diabetes. Arch Biochem Biophys 368:98–104
Beranek M, Novakova D et al (2006) Glycation and advanced glycation end-products in laboratory experiments in vivo and in vitro. Acta Med 49(1):35–39
Stadtman E (1990) Metal catalyzed oxidation of proteins: biochemical mechanism and biological consequences. Free Radic Biol Med 9:315–25
Samuel R, Yernini K, Scott S et al (1999) Novel inhibitors of advanced glycation end products. Biochem Biophys Res Commun 262:651–656
Shenkin A (2006) The key role of micronutrients. Clin Nutr 25:1–13
Sing R, Barden A, Mori T et al (2001) Advanced glycation end products: a review. Diabetologia 44:129–146
Westwood M, McLellan A et al (1994) Receptor-mediated endocytic uptake of methylglyoxal-modified serum albumin. J Biol Chem 269:32293–32298
Yasujiro M, Kazunari Y, Sachico E, Akira H (1995) Protein glycation inhibitors from Thyme (Thymus vulgaris). Biosci Biotechnol Biochem 59:2018–21
Uchida K, Kanematsu M, Sakai K et al (1998) Protein-bound acrolein: potential markers for oxidative stress. Proc Natl Acad Sci USA 95:4882–4887
Bouma B, Kroon-Batenburg MJL, Ya-Ping W (2003) Glycation induces formation of amyloid cross-β structure in albumin. J Biol Chem 278(43):41810–41819
Nagaraj R, Sarkar P, Mally A et al (2002) Effect of pyridoxamine on chemical modification of proteins by carbonyls in diabetic rats: characterization of a major product from the reaction of pyridoxamine and methylglyoxal. Arch Biochem Biophys 402:110–119
Melpomeni P, Jaime U, Helen V (2003) Glucose, advanced glycation end products, and diabetes complications: what is new and what works. Clin diabetes 21:4–7
Rebecca P, Dearlove, Phillip G et al (2008) Inhibition of protein glycation by extracts of culinary herbs and spices. J Med Food 11(2):275–281
Wu C, Yen G (2005) Inhibitory effect of naturally occurring flavonoids on the formation of advanced glycation endproducts. J Agric Food Chem 53:3167–3173
Leopoldini M, Russo N, Chiodo S, Toscano M (2006) Iron chelation by the powerful antioxidant flavonoid quercetin. J Agric Food Chem 54:6343–6351
Metz TO, Alderson NL, Thorpe SR, Baynes JW (2003) Pyridoxamine as a multifunctional pharmaceutical: targeting pathogenic glycation and oxidative damage. Cell Mol Life Sci 62(15):1671–81
Voziyan P, Hudson B (2005) Pyridoxamine: the many virtues of a maillard reaction inhibitor. Ann NY Aca Sci 1043:807–16
Giannoukakis N (2005) Pyridoxamine (BioStratum). Curr Opin Investig Drugs 6(4):410–418
Stadtman E, Levine R (2000) Protein oxidation. Ann NY Aca Sci 889:191–208
Ashley Booth A, Raja Khalifah G et al (1997) In vitro kinetic studies of formation of antigenic advanced glycation end products. J Biol Chem 272:5430–5437
Stankova L, Riddle M, Larned J et al (1984) Plasma ascorbate concentrations and blood cell dehydroascorbate transport in patients with diabetes mellitus. Metabolism 33:347–353
Tarwadi K, Agte V (2004) Linkages of antioxidant, micronutrient and socio-economic status with the degree of oxidative stress and lens opacity in Indian cataract patients. Nutrition 20(3):261–267
Agte V, Nagmote R, Tarwadi K (2004) Comparative in vitro uptake of zinc by erythrocytes of normal vs type 2 diabetic individuals and the associated factors. Diabet Nutr Metab 17:343–349
Vinson A, Howard T (1996) Inhibition of protein glycation and AGEs by ascorbic acid and other vitamins and nutrients. Nutr Biochem 7:659–663
Al-Maroof R, Al-Sharbatti S (2006) Serum zinc levels in diabetic patients and effect of zinc supplementation on glycemic control of type 2 diabetics. Saudi Med J 27:344–350
Tupe R, Agte V (2009) An in vitro study for antiglycation role of zinc along with ascorbic acid and folic acid in BSA glycation. Br J Nutr 103:370–377
Acknowledgements
The authors wish to thank Dr. Rashmi Tupe for her help in glycation experiments. The work presented in this paper is a part of the project funded by the Department of Science and Technology (SR/WOS-A/LS-67/2005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tarwadi, K.V., Agte, V.V. Effect of Micronutrients on Methylglyoxal-Mediated In Vitro Glycation of Albumin. Biol Trace Elem Res 143, 717–725 (2011). https://doi.org/10.1007/s12011-010-8915-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-010-8915-7