Abstract
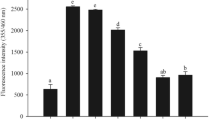
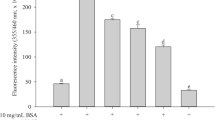
Hyperglycemia in diabetes causes protein glycation that leads to oxidative stress, release of cytokines, and establishment of secondary complications such as neuropathy, retinopathy, and nephropathy. Several other metabolic disorders, stress, and inflammation generate free radicals and oxidative stress. It is essential to study whether oxidative stress independently enhances protein glycation leading to rapid establishment of secondary complications. Oxidative stress was experimentally induced using rotenone and Fenton reagent for in vivo and in vitro studies, respectively. Results showed significant increase in the rate of modification of BSA in the form of fructosamine and protein-bound carbonyls in the presence of fenton reagent. Circular dichroism studies revealed gross structural changes in the reduction of alpha helix structure and decreased protein surface charge was confirmed by zeta potential studies. Use of rotenone demonstrated enhanced AGE formation, ROS generation, and liver and kidney tissue glycation through fluorescence measurement. Similar findings were also observed in cell culture studies. Use of aminoguanidine, a protein glycation inhibitor, demonstrated reduction in these changes; however, a combination of aminoguanidine along with vitamin E demonstrated better amelioration. Thus, oxidative stress accelerates the process of protein glycation causing gross structural changes and tissue glycation in insulin-independent tissues. Use of antioxidants and protein glycation inhibitors in combination are more effective in preventing such changes and could be an effective therapeutic option for preventing establishment of secondary complications of diabetes.




Similar content being viewed by others
Abbreviations
- BSA:
-
Bovine serum albumin
- Fent:
-
Fenton reagent
- Rot:
-
Rotenone
- AMG:
-
Aminoguanidine
- Vit E:
-
Vitamin E
- DCF-DA:
-
2′,7′-Dichlorofluorescin diacetate
- HuH-7:
-
Hepatocellular carcinoma cells
- SOD:
-
Superoxide dismutase
- CAT:
-
Catalase
- PCO:
-
Protein-bound carbonyls
- AGEs:
-
Advanced Glycation End-Products
- TCA:
-
Tricarboxylic acid
- CD:
-
Circular dichroism
References
Turk Z, Ljubic S, Turk N, Benko B (2001) Detection of autoantibodies against advanced glycation end products and AGE-immune complexes in serum of patients with diabetes mellitus. Clin Chim Acta 303:105–115. https://doi.org/10.1016/S0009-8981(00)00389-2
Takeuchi M, Kikuchi S, Sasaki N et al (2004) Involvement of advanced glycation end-products (AGEs) in Alzheimer’s disease. Curr Alzheimer Res 1:39–46. https://doi.org/10.2174/1567205043480582
Vistoli G, De Maddis D, Cipak A et al (2013) Advanced glycoxidation and lipoxidation end products (AGES and ALES): an overview of their mechanisms of formation. Free Radic Res 47:3–27. https://doi.org/10.3109/10715762.2013.815348
Brownlee M (1994) Glycation products and the pathogenesis of diabetic complications. Diabetes 43:836–841. https://doi.org/10.2337/diab.43.6.836
Watkins NG, Thorpe SR, Baynes JW (1985) Glycation of amino groups in protein. Studies on the specificity of modification of RNase by glucose. J Biol Chem 260:10629–10636
Vitek MP, Bhattacharya K, Glendening JM et al (1994) Advanced glycation end products contribute to amyloidosis in Alzheimer disease. Proc Natl Acad Sci USA 91:4766–4770. https://doi.org/10.1073/pnas.91.11.4766
Schmidt MA, Stern DM (2000) RAGE: a new target for the prevention and treatment of the vascular and inflammatory complications of diabetes. Trends Endocrinol Metab 11:368–375. https://doi.org/10.1016/S1043-2760(00)00311-8
Thornalley PJ, Langborg A, Minhas HS (1999) Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem J 344:109–116. https://doi.org/10.1042/bj3440109
Rahbar S, Figarola JL (2003) Novel inhibitors of advanced glycation end products. Arch Biochem Biophys 419:63–79. https://doi.org/10.1016/j.abb.2003.08.009
Brownlee M, Vlassara H, Kooney A et al (1986) Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science 232:1629–1632. https://doi.org/10.1126/science.3487117
Maritim AC, Sanders RA, Watkins JB (2003) Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol 17:24–38. https://doi.org/10.1002/jbt.10058
Rival T, Page RM, Chandraratna DS, Sendall TJ, Ryder E, Liu B et al (2009) Fenton chemistry and oxidative stress mediate the toxicity of the beta-amyloid peptide in a Drosophila model of Alzheimer’s disease. Eur J Neurosci 29:1335–1347. https://doi.org/10.1111/j.1460-9568.2009.06701.x
Omar ME, Abdel-Salam Yasser AK et al (2014) Effect of a single intrastriatal rotenone injection on oxidative stress and neurodegeneration in the rat brain. Comp Clin Pathol 23:1457–1467. https://doi.org/10.1007/s00580-013-1807-4
Li D, Devaraj S, Fuller C, Bucala R, Jialal I (1996) Effect of tocopherol on LDL oxidation and glycation: in vitro and in vivo studies. J Lipid Res 37:1978–1986
Jagdale AD, Bavkar LN et al (2016) Strong inhibition of the polyol pathway diverts glucose flux to protein glycation leading to rapid establishment of secondary complications in diabetes mellitus. J Diabetes Complicat 30:398–405
Johnson RN, Metcalf PA, Baker JR (1983) Fructosamine: a new approach to the estimation of serum glycosylprotein: an index of diabetic control. Clin Chim Acta 127:87–95. https://doi.org/10.1016/0009-8981(83)90078-5
Ansari NA, Ali R (2011) Physicochemical analysis of poly-l-lysine: an insight into the changes induced in lysine residues of proteins on modification with glucose. IUBMB Life 63:26–29. https://doi.org/10.1002/iub.410
Nakamura A, Goto S (1996) Analysis of protein carbonyls with 2, 4-dinitrophenyl hydrazine and its antibodies by immunoblot in two-dimensional gel electrophoresis. J Biochem 119:768–774. https://doi.org/10.1093/oxfordjournals.jbchem.a021306
Kelly SM, Price NC (2000) The use of circular dichroism in the investigation of protein structure and function. Curr Protein Pept Sci 1:349–384. https://doi.org/10.2174/1389203003381315
Joglekar M, Bavkar L, Sistla S, Arvindekar A (2017) Effective inhibition of protein glycation by combinatorial usage of limonene and aminoguanidine through differential and synergistic mechanisms. Int J Biol Macromol 99:563–569. https://doi.org/10.1016/j.ijbiomac.2017.02.104
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Beers RF, Sizer IW (1952) Spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195:133–140
Yeligar SM, Harris FL, Hart CM, Brown LAS (2012) Ethanol induces oxidative stress in alveolar macrophages via upregulation of NADPH oxidases. J Immunol 188:3648–3657. https://doi.org/10.4049/jimmunol.1101278
Nakagawa T, Yokozawa T, Terasawa K, Shu S, Juneja LR (2002) Protective activity of green tea against free radical and glucose-mediated protein damage. J Agric Food Chem 50:2418–2422. https://doi.org/10.1021/jf011339n
Saengkhae C, Loetchutinat C, Garnier-Suillerot A (2003) Kinetic analysis of fluorescein and dihydrofluorescein effluxes in tumour cells expressing the multidrug resistance protein. MRP1. Biochem Pharmacol 65:969–977. https://doi.org/10.1016/S0006-2952(02)01662-3
Edelstein D, Brownlee M (1992) Mechanistic studies of advanced glycation end product inhibition by aminoguanidine. Diabetes 41:26–29. https://doi.org/10.2337/diab.41.1.26
Thornalley PJ (2003) Use of aminoguanidine (pimagedine) to prevent the formation of advanced glycation end products. Arch Biochem Biophys 419:31–40. https://doi.org/10.1016/j.abb.2003.08.013
Goh SY, Cooper ME (2008) The role of advanced glycation end products in progression and complications of diabetes. J Clin Endocrinol Metab 93:1143–1152. https://doi.org/10.1210/jc.2007-1817
Meerwaldt R, Links T, Zeebregts C, Tio T, Hillebrands J, Smit A (2008) The clinical relevance of assessing advanced glycation end products accumulation in diabetes. Cardiovasc Diabetol 7:29. https://doi.org/10.1186/1475-2840-7-29
Vlassara H, Uribarri J (2014) Advanced glycation end products (AGE) and diabetes: cause, effect, or both? Curr Diab Rep 14:453. https://doi.org/10.1007/s11892-013-0453-1
Awasthi S, Saraswathi NT (2016) Non-enzymatic glycation mediated structure-function changes in proteins: case of serum albumin. Rsc Adv 6:90739–90753. https://doi.org/10.1039/C6RA08283A
Greifenhagen U, Frolov A, Blüher M, Hoffmann R (2016) Site-specific analysis of advanced glycation end products in plasma proteins of type 2 diabetes mellitus patients. Anal Bioanal Chem 408:5557–5566. https://doi.org/10.1007/s00216-016-9651-4
Miyata T, Kurokawa K, De Strihou CVY (2000) Advanced glycation and lipoxidation end products: role of reactive carbonyl compounds generated during carbohydrate and lipid metabolism. J Am Soc Nephrol 11:1744–1752
Hunt JV, Wolff SP (1991) Oxidative glycation and free radical production: a causal mechanism of diabetic complications. Free Radic Res Commun 1:115–123. https://doi.org/10.3109/10715769109145775
Aronson D, Rayfield EJ (2008) How hyperglycemia promotes atherosclerosis: molecular mechanisms. Cardiovasc Diabetol 2:89. https://doi.org/10.1186/1475-2840-1-1
Verdile G, Keane KN, Cruzat VF et al (2015) Inflammation and oxidative stress: the molecular connectivity between insulin resistance, obesity, and Alzheimer’s disease. Mediat Inflamm 2:89. https://doi.org/10.1155/2015/105828
Namioka N, Hanyu H, Hirose D et al (2017) Oxidative stress and inflammation are associated with physical frailty in patients with Alzheimer’s disease. Geriatr Geriatr Gerontol Int 17:913–918. https://doi.org/10.1111/ggi.12804
Plucińska K, Dekeryte R, Koss D et al (2016) Neuronal human BACE1 knockin induces systemic diabetes in mice. Diabetologia 59:1513–1523. https://doi.org/10.1007/s00125-016-3960-1
Kanti Das T, Wati MR, Fatima-Shad K (2015) Oxidative stress gated by Fenton and Haber Weiss reactions and its association with Alzheimer’s disease. Arch Neurosci 2:e20078. https://doi.org/10.1007/s00125-016-3960-1
Patil RS, Jagdale AD, Nalawade ML et al (2018) Glycation inhibitors and probiotics can ameliorate the changes caused by high fructose feed. Int J Pharm Pharm Sci 10:28–32. https://doi.org/10.22159/ijpps.2018v10i7.26870
Acknowledgements
Laxman Naghnath Bavkar acknowledges the University Grant Commission (UGC), New Delhi, India for a fellowship under the Special Assistance Program for Basic Scientific Research. Rahul Shivaji Patil acknowledges the Department of Science and Technology (DST), New Delhi, India for DST-INSPIRE fellowship. Chemicals and research work were supported by RUSA, Government of Maharashtra funded to Prof. (Mrs.) A. U. Arvindekar. The authors are thankful to the Department of Biochemistry, Shivaji University, Kolhapur for providing work place and laboratory.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bavkar, L.N., Patil, R.S., Rooge, S.B. et al. Acceleration of protein glycation by oxidative stress and comparative role of antioxidant and protein glycation inhibitor. Mol Cell Biochem 459, 61–71 (2019). https://doi.org/10.1007/s11010-019-03550-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-019-03550-7