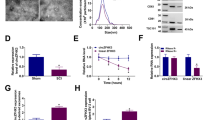
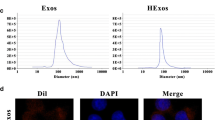
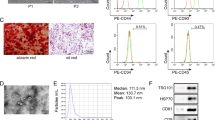
Abstract
Spinal cord injury (SCI) is a highly debilitating disorder of the central nervous system that can severely impact an affected patient’s quality of life. This study aimed to examine how adipose-derived mesenchymal stem cell exosomes (ADSC-exos) can be used to treat spinal cord injury. We analysed differentially expressed mRNAs in SCI using bioinformatics data, gene expression profiles in inflammatory cell models, RT-qPCR and WB. Apoptosis was detected with flow cytometry. Starbase provides the control mechanism for FDFT1. Target interactions were detected with dual-luciferase reporter and RIP assays. Exosomes were isolated from adipose tissue-derived mesenchymal stem cells and subsequently characterized with western blot analysis, transmission electron microscopy and nanoparticle tracking analysis. By analysing the GSE102964 database, we found that FDFT1 was significantly downregulated as SCI progressed. Overexpression of FDFT1 can significantly reverse the inflammatory response and apoptosis of BV2 cells induced by hemin. Mechanically, ADSC-exos can affect the expression of FDFT1 through the ceRNA mechanism mediated by LRRC75A-AS1 and in an RBP-dependent manner mediated by IGF2BP2. The overexpression of LRRC75A-AS1 significantly enhances BV2 apoptosis and can be reversed by FDFT1 knockdown. ADSC-exos LRRC75A-AS1 inhibits inflammation and reduces SCI by increasing the expression and stability of FDFT1 mRNA in a ceRNA and RBP-dependent manner.





Similar content being viewed by others
Data Availability
The datasets used and/or analysed in the current study are available.
Abbreviations
- SCI:
-
Spinal cord injury
- FDFT1:
-
Farnesyl-diphosphate farnesyltransferase 1
- ADSCs:
-
Adipose mesenchymal stem cells
- CNS:
-
The central nervous system
- MSCs:
-
Mesenchymal stem cells
- DEGs:
-
Differentially expressed genes
- GEO:
-
Gene Expression Omnibus
- PPI:
-
Protein-protein interaction
- GO:
-
Gene Ontology
- NTA:
-
Nanoparticle tracking analysis
- NO:
-
Nitric oxide
- TEM:
-
Transmission electron microscope
- ADSC-exos:
-
Adipose-derived mesenchymal stem cell exosomes
References
Choi, J. H., Park, P. J., Din, V., Sam, N., Iv, V., & Park, K. B. (2017). Epidemiology and clinical management of traumatic spine injuries at a major government hospital in Cambodia. Asian Spine Journal, 11(6), 908–916. https://doi.org/10.4184/asj.2017.11.6.908
Yeo, R. W., Lai, R. C., Zhang, B., Tan, S. S., Yin, Y., Teh, B. J., & Lim, S. K. (2013). Mesenchymal stem cell: An efficient mass producer of exosomes for drug delivery. Advanced Drug Delivery Reviews, 65(3), 336–341. https://doi.org/10.1016/j.addr.2012.07.001
Schwab, J. M., Chiang, N., Arita, M., & Serhan, C. N. (2007). Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature, 447(7146), 869–874. https://doi.org/10.1038/nature05877
Nakkala, J. R., Li, Z., Ahmad, W., Wang, K., & Gao, C. (2021). Immunomodulatory biomaterials and their application in therapies for chronic inflammation-related diseases. Acta Biomaterialia, 123, 1–30. https://doi.org/10.1016/j.actbio.2021.01.025
DiSabato, D. J., Quan, N., & Godbout, J. P. (2016). Neuroinflammation: The devil is in the details. Journal Of Neurochemistry, 139(Suppl 2), 136–153. https://doi.org/10.1111/jnc.13607
Fleming, J. C., Norenberg, M. D., Ramsay, D. A., Dekaban, G. A., Marcillo, A. E., Saenz, A. D., Pasquale-Styles, M., Dietrich, W. D., & Weaver, L. C. (2006). The cellular inflammatory response in human spinal cords after injury. Brain : A Journal Of Neurology, 129(Pt 12), 3249–3269. https://doi.org/10.1093/brain/awl296
Lee, Y. B., Yune, T. Y., Baik, S. Y., Shin, Y. H., Du, S., Rhim, H., Lee, E. B., Kim, Y. C., Shin, M. L., Markelonis, G. J., & Oh, T. H. (2000). Role of tumor necrosis factor-alpha in neuronal and glial apoptosis after spinal cord injury. Experimental Neurology, 166(1), 190–195. https://doi.org/10.1006/exnr.2000.7494
Wilson, J. R., Forgione, N., & Fehlings, M. G. (2013). Emerging therapies for acute traumatic spinal cord injury. Cmaj : Canadian Medical Association Journal = Journal De L'association Medicale Canadienne, 185(6), 485–492. https://doi.org/10.1503/cmaj.121206
Gao, L., Peng, Y., Xu, W., He, P., Li, T., Lu, X., & Chen, G. (2020). Progress in stem cell therapy for spinal cord injury. Stem Cells International, 2020, 2853650. https://doi.org/10.1155/2020/2853650
Lu, X. C., Zheng, J. Y., Tang, L. J., Huang, B. S., Li, K., Tao, Y., Yu, W., Zhu, R. L., Li, S., & Li, L. X. (2015). MiR-133b promotes neurite outgrowth by targeting RhoA expression. Cellular Physiology And Biochemistry : International Journal Of Experimental Cellular Physiology, Biochemistry, And Pharmacology, 35(1), 246–258. https://doi.org/10.1159/000369692
Xia, J., Minamino, S., Kuwabara, K., & Arai, S. (2019). Stem cell secretome as a new booster for regenerative medicine. Bioscience Trends, 13(4), 299–307. https://doi.org/10.5582/bst.2019.01226
Lv, K., Li, Q., Zhang, L., Wang, Y., Zhong, Z., Zhao, J., Lin, X., Wang, J., Zhu, K., Xiao, C., Ke, C., Zhong, S., Wu, X., Chen, J., Yu, H., Zhu, W., Li, X., Wang, B., Tang, R., et al. (2019). Incorporation of small extracellular vesicles in sodium alginate hydrogel as a novel therapeutic strategy for myocardial infarction. Theranostics, 9(24), 7403–7416. https://doi.org/10.7150/thno.32637
Lässer, C., Jang, S. C., & Lötvall, J. (2018). Subpopulations of extracellular vesicles and their therapeutic potential. Molecular Aspects Of Medicine, 60, 1–14. https://doi.org/10.1016/j.mam.2018.02.002
Shao, M., Jin, M., Xu, S., Zheng, C., Zhu, W., Ma, X., & Lv, F. (2020). Exosomes from long noncoding RNA-Gm37494-ADSCs repair spinal cord injury via shifting microglial M1/M2 polarization. Inflammation, 43(4), 1536–1547. https://doi.org/10.1007/s10753-020-01230-z
Zhao, Y., Chen, Y., Wang, Z., Xu, C., Qiao, S., Liu, T., Qi, K., Tong, D., & Li, C. (2022). Bone marrow mesenchymal stem cell exosome attenuates inflammasome-related pyroptosis via delivering circ_003564 to improve the recovery of spinal cord injury. Molecular Neurobiology, 59(11), 6771–6789. https://doi.org/10.1007/s12035-022-03006-y
Luo, Y., Xu, T., Liu, W., Rong, Y., Wang, J., Fan, J., Yin, G., & Cai, W. (2021). Exosomes derived from GIT1-overexpressing bone marrow mesenchymal stem cells promote traumatic spinal cord injury recovery in a rat model. The International Journal Of Neuroscience, 131(2), 170–182. https://doi.org/10.1080/00207454.2020.1734598
Wang, Z., Song, Y., Han, X., Qu, P., & Wang, W. (2020). Long noncoding RNA PTENP1 affects the recovery of spinal cord injury by regulating the expression of miR-19b and miR-21. Journal Of Cellular Physiology, 235(4), 3634–3645. https://doi.org/10.1002/jcp.29253
Ryan, K. K., Woods, S. C., & Seeley, R. J. (2012). Central nervous system mechanisms linking the consumption of palatable high-fat diets to the defense of greater adiposity. Cell Metabolism, 15(2), 137–149. https://doi.org/10.1016/j.cmet.2011.12.013
Saunders, L. L., Clarke, A., Tate, D. G., Forchheimer, M., & Krause, J. S. (2015). Lifetime prevalence of chronic health conditions among persons with spinal cord injury. Archives Of Physical Medicine And Rehabilitation, 96(4), 673–679. https://doi.org/10.1016/j.apmr.2014.11.019
Selassie, A., Snipe, L., Focht, K. L., & Welldaregay, W. (2013). Baseline prevalence of heart diseases, hypertension, diabetes, and obesity in persons with acute traumatic spinal cord injury: Potential threats in the recovery trajectory. Topics In Spinal Cord Injury Rehabilitation, 19(3), 172–182. https://doi.org/10.1310/sci1903-172
Bai, G., Jiang, L., Meng, P., Li, J., Han, C., Wang, Y., & Wang, Q. (2021). LncRNA Neat1 promotes regeneration after spinal cord injury by targeting miR-29b. Journal Of Molecular Neuroscience : Mn, 71(6), 1174–1184. https://doi.org/10.1007/s12031-020-01740-3
Zhou, J., Li, Z., Wu, T., Zhao, Q., Zhao, Q., & Cao, Y. (2020). LncGBP9/miR-34a axis drives macrophages toward a phenotype conducive for spinal cord injury repair via STAT1/STAT6 and SOCS3. Journal Of Neuroinflammation, 17(1), 134. https://doi.org/10.1186/s12974-020-01805-5
Wu, J., Li, X., Wang, Q., Wang, S., He, W., Wu, Q., & Dong, C. (2022). LncRNA/miRNA/mRNA ceRNA network analysis in spinal cord injury rat with physical exercise therapy. PeerJ, 10, e13783. https://doi.org/10.7717/peerj.13783
Kwan, T., Floyd, C. L., Kim, S., & King, P. H. (2017). RNA binding protein human antigen R Is translocated in astrocytes following spinal cord injury and promotes the inflammatory response. Journal Of Neurotrauma, 34(6), 1249–1259. https://doi.org/10.1089/neu.2016.4757
Wang, D., Xu, X., Pan, J., Zhao, S., Li, Y., Wang, Z., Yang, J., Zhang, X., Wang, Y., & Liu, M. (2021). GAS5 knockdown alleviates spinal cord injury by reducing VAV1 expression via RNA binding protein CELF2. Scientific Reports, 11(1), 3628. https://doi.org/10.1038/s41598-021-83145-9 (Retraction published Sci Rep. 2022 Dec 20;12(1):21991).
Xiang, W., Jiang, L., Zhou, Y., Li, Z., Zhao, Q., Wu, T., Cao, Y., & Zhou, J. (2021). The lncRNA Ftx/miR-382-5p/Nrg1 axis improves the inflammation response of microglia and spinal cord injury repair. Neurochemistry International, 143, 104929. https://doi.org/10.1016/j.neuint.2020.104929
Li, D., Zhang, P., Yao, X., Li, H., Shen, H., Li, X., Wu, J., & Lu, X. (2018). Exosomes derived from miR-133b-modified mesenchymal stem cells promote recovery after spinal cord injury. Frontiers In Neuroscience, 12, 845. https://doi.org/10.3389/fnins.2018.00845
Shin, K. O., Ha, D. H., Kim, J. O., Crumrine, D. A., Meyer, J. M., Wakefield, J. S., Lee, Y., Kim, B., Kim, S., Kim, H. K., Lee, J., Kwon, H. H., Park, G. H., Lee, J. H., Lim, J., Park, S., Elias, P. M., Park, K., Yi, Y. W., & Cho, B. S. (2020). Exosomes from human adipose tissue-derived mesenchymal stem cells promote epidermal barrier repair by inducing de novo synthesis of ceramides in atopic dermatitis. Cells, 9(3), 680. https://doi.org/10.3390/cells9030680
Huang, S., Ge, X., Yu, J., Han, Z., Yin, Z., Li, Y., Chen, F., Wang, H., Zhang, J., & Lei, P. (2018). Increased miR-124-3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons. Faseb Journal : Official Publication Of The Federation Of American Societies For Experimental Biology, 32(1), 512–528. https://doi.org/10.1096/fj.201700673R
Lu, T., Peng, W., Liang, Y., Li, M., Li, D. S., Du, K. H., Zhu, J. H., & Wu, J. H. (2020). PTEN-silencing combined with ChABC-overexpression in adipose-derived stem cells promotes functional recovery of spinal cord injury in rats. Biochemical And Biophysical Research Communications, 532(3), 420–426. https://doi.org/10.1016/j.bbrc.2020.08.085
Ji, W., Jiang, W., Li, M., Li, J., & Li, Z. (2019). miR-21 deficiency contributes to the impaired protective effects of obese rat mesenchymal stem cell-derived exosomes against spinal cord injury. Biochimie, 167, 171–178. https://doi.org/10.1016/j.biochi.2019.10.002
Zhang, J., Li, S., Zhang, L., Xu, J., Song, M., Shao, T., Huang, Z., & Li, Y. (2020). RBP EIF2S2 promotes tumorigenesis and progression by regulating MYC-mediated inhibition via FHIT-related enhancers. Molecular Therapy : The Journal Of The American Society Of Gene Therapy, 28(4), 1105–1118. https://doi.org/10.1016/j.ymthe.2020.02.004
Guo, X. D., He, X. G., Yang, F. G., Liu, M. Q., Wang, Y. D., Zhu, D. X., Zhang, G. Z., Ma, Z. J., & Kang, X. W. (2021). Research progress on the regulatory role of microRNAs in spinal cord injury. Regenerative Medicine, 16(5), 465–476. https://doi.org/10.2217/rme-2020-0125
Hurlbert, R. J., Hadley, M. N., Walters, B. C., Aarabi, B., Dhall, S. S., Gelb, D. E., Rozzelle, C. J., Ryken, T. C., & Theodore, N. (2015). Pharmacological therapy for acute spinal cord injury. Neurosurgery, 76(Suppl 1), S71–S83. https://doi.org/10.1227/01.neu.0000462080.04196.f7
Zhaohui, C., & Shuihua, W. (2020). Protective effects of SIRT6 against inflammation, oxidative stress, and cell apoptosis in spinal cord injury. Inflammation, 43(5), 1751–1758. https://doi.org/10.1007/s10753-020-01249-2
Chen, J., Wang, Z., Zheng, Z., Chen, Y., Khor, S., Shi, K., He, Z., Wang, Q., Zhao, Y., Zhang, H., Li, X., Li, J., Yin, J., Wang, X., & Xiao, J. (2017). Neuron and microglia/macrophage-derived FGF10 activate neuronal FGFR2/PI3K/Akt signaling and inhibit microglia/macrophages TLR4/NF-κB-dependent neuroinflammation to improve functional recovery after spinal cord injury. Cell death & disease, 8(10), e3090. https://doi.org/10.1038/cddis.2017.490
Kertmen, H., Celikoglu, E., Ozturk, O. C., Gürer, B., Bozkurt, H., Kanat, M. A., Arikok, A. T., Erguder, B. I., Sargon, M. F., & Sekerci, Z. (2018). Comparative effects of methylprednisolone and tetracosactide (ACTH1-24) on ischemia/reperfusion injury of the rabbit spinal cord. Archives Of Medical Science : Ams, 14(6), 1459–1470. https://doi.org/10.5114/aoms.2017.65650
Fehlings, M. G., Wilson, J. R., & Cho, N. (2014). Methylprednisolone for the treatment of acute spinal cord injury: Counterpoint. Neurosurgery, 61(Suppl 1), 36–42. https://doi.org/10.1227/NEU.0000000000000412
Dos Santos, E., Santos, C., Welch, B. A., Edwards, S. R., Harris, K. K., Duncan, B. C., Himel, A. R., & Grayson, B. E. (2022). Immune and metabolic biomarkers in a rodent model of spinal cord contusion. Global Spine Journal, 12(1), 110–120. https://doi.org/10.1177/2192568220950337
Ding, J., Reynolds, L. M., Zeller, T., Müller, C., Lohman, K., Nicklas, B. J., Kritchevsky, S. B., Huang, Z., de la Fuente, A., Soranzo, N., Settlage, R. E., Chuang, C. C., Howard, T., Xu, N., Goodarzi, M. O., Chen, Y. D., Rotter, J. I., Siscovick, D. S., Parks, J. S., et al. (2015). Alterations of a cellular cholesterol metabolism network are a molecular feature of obesity-related type 2 diabetes and cardiovascular disease. Diabetes, 64(10), 3464–3474. https://doi.org/10.2337/db14-1314
Chen, G., Fang, X., & Yu, M. (2015). Regulation of gene expression in rats with spinal cord injury based on microarray data. Molecular Medicine Reports, 12(2), 2465–2472. https://doi.org/10.3892/mmr.2015.3670
Xue, Y. H., & Ge, Y. Q. (2020). Construction of lncRNA regulatory networks reveal the key lncRNAs associated with pituitary adenomas progression. Mathematical Biosciences And Engineering : Mbe, 17(3), 2138–2149. https://doi.org/10.3934/mbe.2020113
Hou, P., Meng, S., Li, M., Lin, T., Chu, S., Li, Z., Zheng, J., Gu, Y., & Bai, J. (2021). LINC00460/DHX9/IGF2BP2 complex promotes colorectal cancer proliferation and metastasis by mediating HMGA1 mRNA stability depending on m6A modification. Journal Of Experimental & Clinical Cancer Research : Cr, 40(1), 52. https://doi.org/10.1186/s13046-021-01857-2
Mukhamedshina, Y. O., Akhmetzyanova, E. R., Kostennikov, A. A., Zakirova, E. Y., Galieva, L. R., Garanina, E. E., Rogozin, A. A., Kiassov, A. P., & Rizvanov, A. A. (2018). Adipose-derived mesenchymal stem cell application combined with fibrin matrix promotes structural and functional recovery following spinal cord injury in rats. Frontiers In Pharmacology, 9, 343. https://doi.org/10.3389/fphar.2018.00343
Li, N., & Hua, J. (2017). Interactions between mesenchymal stem cells and the immune system. Cellular And Molecular Life Sciences : Cmls, 74(13), 2345–2360. https://doi.org/10.1007/s00018-017-2473-5
Gao, L., Xu, W., Li, T., Chen, J., Shao, A., Yan, F., & Chen, G. (2018). Stem cell therapy: A promising therapeutic method for intracerebral hemorrhage. Cell Transplantation, 27(12), 1809–1824. https://doi.org/10.1177/0963689718773363
Kim, H. Y., Kim, T. J., Kang, L., Kim, Y. J., Kang, M. K., Kim, J., Ryu, J. H., Hyeon, T., Yoon, B. W., Ko, S. B., & Kim, B. S. (2020). Mesenchymal stem cell-derived magnetic extracellular nanovesicles for targeting and treatment of ischemic stroke. Biomaterials, 243, 119942. https://doi.org/10.1016/j.biomaterials.2020.119942
Przekora, A., & Juszkiewicz, L. (2020). The effect of autologous adipose tissue-derived mesenchymal stem cells’ therapy in the treatment of chronic posttraumatic spinal cord injury in a domestic ferret patient. Cell Transplantation, 29, 963689720928982. https://doi.org/10.1177/0963689720928982
Tang, L., Lu, X., Zhu, R., Qian, T., Tao, Y., Li, K., Zheng, J., Zhao, P., Li, S., Wang, X., & Li, L. (2016). Adipose-derived stem cells expressing the neurogenin-2 promote functional recovery after spinal cord injury in rat. Cellular And Molecular Neurobiology, 36(5), 657–667. https://doi.org/10.1007/s10571-015-0246-y
Shen, H., Yao, X., Li, H., Li, X., Zhang, T., Sun, Q., Ji, C., & Chen, G. (2018). Role of exosomes derived from miR-133b modified MSCs in an experimental rat model of intracerebral hemorrhage. Journal Of Molecular Neuroscience : Mn, 64(3), 421–430. https://doi.org/10.1007/s12031-018-1041-2
Chang, C. L., Sung, P. H., Chen, K. H., Shao, P. L., Yang, C. C., Cheng, B. C., Lin, K. C., Chen, C. H., Chai, H. T., Chang, H. W., Yip, H. K., & Chen, H. H. (2018). Adipose-derived mesenchymal stem cell-derived exosomes alleviate overwhelming systemic inflammatory reaction and organ damage and improve outcome in rat sepsis syndrome. American Journal Of Translational Research, 10(4), 1053–1070.
He, L., Zhu, C., Jia, J., Hao, X. Y., Yu, X. Y., Liu, X. Y., & Shu, M. G. (2020). ADSC-exos containing MALAT1 promotes wound healing by targeting miR-124 through activating Wnt/β-catenin pathway. Bioscience Reports, 40(5), BSR20192549. https://doi.org/10.1042/BSR20192549
Shao, X., Qin, J., Wan, C., Cheng, J., Wang, L., Ai, G., Cheng, Z., & Tong, X. (2021). ADSC exosomes mediate lncRNA-MIAT alleviation of endometrial fibrosis by regulating miR-150-5p. Frontiers In Genetics, 12, 679643. https://doi.org/10.3389/fgene.2021.679643
Funding
This work was financially supported by the National Natural Science Foundation of China (grant number 81701159), the Taishan Scholar Project of Shandong Province of China (grant number tsqn202103200), Shandong Natural Science Foundation General Project (grant number ZR2021MH303) and the Youth Scientific Research Fund of Liaocheng People’s Hospital (grant number 201910915).
Author information
Authors and Affiliations
Contributions
Conceptualization: XhX. Data curation and formal analysis: PX. Investigation: XyX. Methodology: ZX and ZL. Project administration and resources: ZH. Supervision: XL and YX. Writing—original draft: XyX, XhX and Peng Xu. Writing—review and editing: XL and YX.
Corresponding authors
Ethics declarations
Ethics Approval
Animal experiments were conducted according to the Chinese Animal Welfare Act and Guidance for Animal Experimentation of Liaocheng People’s Hospital. The study protocol was approved by the Ethics Committee of the Liaocheng People’s Hospital (Protocol No.: 2021076).
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xing, X., Xu, P., Xing, X. et al. Effects of ADSC-Derived Exosome LRRC75A-AS1 on Anti-inflammatory Function After SCI. Appl Biochem Biotechnol (2024). https://doi.org/10.1007/s12010-023-04836-9
Accepted:
Published:
DOI: https://doi.org/10.1007/s12010-023-04836-9