Abstract
Remdesivir (REM) and dexamethasone (DEX) both have been used to treat coronavirus disease 2019 (COVID-19). The present study aimed to evaluate the effects of REM and DEX on kidney structure and function with particular focus on the probable renal sirtuin-1 (SIRT1) expression alteration in rats. Twenty-four male Wistar rats were divided into four groups, as follows: group A (control) received normal saline (5 mL/kg/day for 10 days); group B (REM) received REM (17 mg/kg/day on the first day, and 8.5 mg/kg/day on the 2nd–10th days); group C (REM + DEX) received both REM (17 mg/kg/day on the first day, and 8.5 mg/kg/day on the 2nd–10th days) and DEX (7 mg/kg/day, for 10 days); group D (DEX) received DEX (7 mg/kg/day for 10 days). Renal SIRT1 expression and kidney structure and function-related factors were evaluated by standard methods. The mean levels of urea in the REM + DEX group (60.83 ± 6.77, mg/dL) were significantly higher than in the control (48.33 ± 3.01, mg/dL; p = 0.002) and DEX (51.22 ± 4.99, mg/dL; p = 0.018) groups. The mean levels of creatinine in the REM (0.48 ± 0.08, mg/dL) and REM + DEX (0.50 ± 0.04, mg/dL) groups were higher than in the control group (48.33 ± 3.0 mg/dL) significantly (p = 0.022 and p = 0.010, respectively). The renal SIRT1 expression was significantly (p = 0.018) lower in the REM + DEX group (0.36 ± 0.35) than in the control group (1.34 ± 0.48). Tubulointerstitial damage (TID) scores in REM + DEX-treated rats (2.60 ± 0.24) were significantly higher than in the control (0.17 ± 0.17, p = 0.001) and DEX (0.50 ± 0.29, p = 0.005) groups. The administration of DEX and REM might lead to kidney injury associated with SIRT1 downregulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
December 2019 was the emergence of the new coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), causing the coronavirus disease 2019 (COVID-19) pandemic [1]. Although no drug has been developed to treat this disease definitively, some agents have been introduced for earlier recovery of the patients [2]. Remdesivir (REM) is a viral RNA polymerase inhibitor drug which has been approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of COVID-19 under an emergency use authorization [3]. On the other hand, the World Health Organization (WHO) does not recommend the use of this drug for the treatment of COVID-19 as it has no statistically significant effect on the mortality rate in a large, simple, international, open-label randomized trial. However, several countries continue to apply REM in the treatment of COVID-19 [3, 4]. Dexamethasone (DEX), a long-acting glucocorticoid receptor agonist with anti-inflammatory effects, has also been shown to be influential in reducing COVID-19 mortality [5, 6]. In addition to the effectiveness, the safety of REM and DEX should be concerned [4] and they may be associated with some side effects [4, 7, 8]. Renal and urinary injuries had disproportionately higher reporting with REM as a suspect drug compared with other drugs [4, 9]. However, in a study by Yin et al. [10], it was showed that REM ameliorated acute kidney injury by inhibiting inflammatory immune responses. Therefore, the effects of these drugs on the kidneys and underlying mechanisms of action have not yet been fully understood with contradictory results.
Kidneys express sirtuin-1 (SIRT1), a member of the sirtuins family, a group of nicotinamide adenine dinucleotide-dependent histone deacetylases, which regulates various biological pathways such as cellular energy metabolism, mitochondrial biogenesis, stress response, apoptosis, inflammation, and fibrosis [11]. SIRT1 has been shown to protect kidneys against ischemic or toxic substance injuries and improve renal function [12]. The present study aimed to evaluate the effects of REM on kidney structure and function with particular focus on the probable renal SIRT1 expression alteration in rats.
Materials and Methods
Animals
In the present study, 24 male 12-week-old Wistar rats weighing 220 to 280 g were purchased from the Pasteur Institute of Iran (Tehran, Iran). The animal protocol was according to the Guide for Care and Use of Laboratory Animals (US Department of Health, Education, and Welfare (DHEW), Publication Number 78–23, National Institutes of Health (NIH), revised 1978), and local guidelines for compassionate use of animals in research. The animals were housed two per cage and provided free access to tap water and standard chow. The animals were kept in similar laboratory conditions (18 to 23 °C room temperature and controlled humidity) with alternating 12-h light and dark cycles.
Group Design (Drug Treatment)
After a 2-week acclimation period, the weight-matched rats were randomly allocated into four groups (six rats per group):
Group A (control) received daily intraperitoneal injections of normal saline (0.9% sodium chloride solution; Iran Injectable and Pharmaceutical Products Co., Tehran, Iran) in a dose of 5 mL/kg body weight for 10 days.
Group B (REM) received daily intraperitoneal injections of REM (Rooyan Darou Pharmaceutical Co., Tehran, Iran) at a dose of 17 mg/kg on the first day, and 8.5 mg/kg from the second to 10th days of administration [13].
Group C (REM + DEX) received both REM (17 mg/kg/day on the first day, and 8.5 mg/kg/day from the second to 10th days of administration; intraperitoneal injection) and dexamethasone (DEX) (Iran Hormone, Tehran, Iran; 7 mg/kg/day; intramuscular injection for 10 days).
Group D (DEX) received daily intramuscular injections of DEX (7 mg/kg) for 10 days.
After the administration period, all the rats were weighed and anaesthetized by ketamine (60 mg/kg) and xylazine (10 mg/kg) intraperitoneally. Intravenous blood samples were taken and the sera were separated by centrifugation at 3000 g (unit of gravity) for 15 min and stored at − 80 °C. Thereafter, the rats were killed with a single lethal dose (200 mg/kg) of ketamine. The kidneys were excised and weighed separately. The right kidneys were collected and fixed in 10% formalin for histological assessments. The left kidneys were snap-frozen in liquid nitrogen and stored at − 80 °C until use.
Determination of Serum Parameters
The serum concentrations of urea and creatinine (Cr) were evaluated colorimetrically using commercial reagents in an automated chemical analyzer (Roche Cobas Mira) to assess kidney function.
Analysis of Renal SIRT1 mRNA Expression
Total RNA was extracted from the kidney tissues using Trizol reagent (Roche, Basel, Switzerland) according to the manufacturer’s instructions. Briefly, the tissue was frozen immediately and ground to a fine powder under liquid nitrogen. Then, total RNA was extracted and purified [14], and its concentration was quantified on a DS-11 FX spectrophotometer (DeNovix, Wilmington, USA). One microgram of the total RNA was utilized for complementary DNA (cDNA) synthesis by Oligo (dT) primers using the easy cDNA Synthesis kit (Pars Tous Biotechnology, Iran) based on the manufacturer’s instruction.
Quantitative real-time PCR analysis was conducted in duplicate utilizing SYBR Green I 2 × Master Mix (Pars Tous Biotechnology, Iran) on an ABI 7500 FAST real-time PCR system (Applied Biosystems, CA, USA). Results were normalized to the gene expression of rat glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as the reference gene (the specific primer sequences are depicted in Table 1). PCR program comprised a preincubation step at 94 °C for 10 min, followed by 40 cycles of denaturation (94 °C, 10 s), annealing (60 °C, 30 s), and extension (72 °C, 20 s) steps with the final extension step at 72 °C for 10 min. The 2 − ΔΔCt formula was used to calculate the gene expression ratio of SIRT1 to GAPDH with respect to the control group.
Histological Assessment
Formalin-fixed kidney samples were gradually dehydrated in the ascending grades of ethanol and were then kept in xylene overnight for complete dehydration. They were embedded in paraffin, and each 5µ section stained with hematoxylin and eosin. Ten areas at a 200-fold magnification of each slide were analyzed by a pathologist blinded to the groups under a light microscope.
The slides were then scored semi-quantitatively (0 to 3) [15] according to the severity of pathological changes for evaluation of tubulointerstitial damage (TID). They included 0 (no changes), 1 (less than 25% of the field was changed), 2 (25–50% of the area was changed), and 3 (more than 50% of the area was changed). The mean value of the evaluated scores on each slide was recorded.
Statistical Analysis
Initially, the variables were statistically checked for normality by the one-sample Kolmogorov–Smirnov test. All variables had normal distributions and therefore were presented as mean ± standard deviation (SD) except TID scores which were presented as mean ± standard error (SE). Statistical comparisons of the groups were performed by one-way analysis of variance (ANOVA) and Bonferroni’s post-hoc analysis. Pearson’s correlation coefficient was also calculated. Statistical significance was set at a p-value of less than 0.05. The analyses were carried out in SPSS 16.0 software.
Results
Basic Parameters
The data of body weight (BW), urea, and Cr variables are presented in Table 2. All the rats were weight-matched before the intervention (p > 0.05), but after the intervention, group C (REM + DEX) had significantly lower mean BW (160.75 ± 13.15, g) than groups A (control) (255.83 ± 23.69, g) and B (REM) (229.83 ± 27.51, g) (p < 0.001). The BW mean in group D (DEX) (171.50 ± 11.12, g) was also significantly lower than in the control (255.83 ± 23.69, g; p < 0.001) and REM (229.83 ± 23.69, g; p = 0.002) groups.
The mean level of urea in the REM + DEX group (60.83 ± 6.77, mg/dL) was significantly higher than that in the control (48.33 ± 3.01, mg/dL; p = 0.002) and DEX (51.22 ± 4.99, mg/dL; p = 0.018) groups.
The mean levels of Cr in REM (0.48 ± 0.08, mg/dL) and REM + DEX (0.50 ± 0.04, mg/dL) groups were significantly (p = 0.022, p = 0.010, respectively) higher than in the control group (0.37 ± 0.06, mg/dL).
Renal Expression of SIRT1
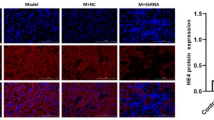
The results of real-time PCR showed that mRNA expression of renal SIRT1 was significantly (p = 0.018) lower in the REM + DEX group (0.36 ± 0.35) than in the control group (1.34 ± 0.48) (Fig. 1). There was no other significant difference in renal SIRT1 expression between the groups.
Renal mRNA expression of SIRT1 in the studied groups. The results of real-time PCR showed that mRNA expressions of renal SIRT1 were significantly (p = 0.018) lower in the REM + DEX group (0.36 ± 0.35) than in the control group (1.34 ± 0.48). There was no other significant difference in renal SIRT1 expression between the groups
Histological Findings
The administration of REM and DEX led to increased TID which was exhibited by histological alteration including hyperemia, inflammatory cell infiltration, tubular atrophy, and vacuolization. As illustrated in Fig. 2, the TID score (2.60 ± 0.24) in REM + DEX-treated rats was significantly higher than in the control (0.17 ± 0.17, p = 0.001) and DEX (0.50 ± 0.29, p = 0.005) groups.
Representative histologic findings. Hematoxylin and eosin (H&E) staining of the rats’ kidney tissues in the four studied groups (magnification, × 200) showed that the administration of remdesivir (REM) and dexamethasone (DEX) led to increased tubulointerstitial damage (TID) which were exhibited by histological alteration including hyperemia (asterisks), inflammatory cell infiltration (arrows), tubular atrophy (arrowhead), and vacuolization (dashed arrow). The TID score (2.60 ± 0.24) in REM + DEX-treated rats was significantly higher than in the control (0.17 ± 0.17, p = 0.001) and DEX (0.50 ± 0.29, p = 0.005) groups
Relationships Among SIRT1, Urea, Cr, and TID Score
Figure 3 shows the correlations between the main evaluated parameters. As shown in Fig. 3 A and C, renal SIRT1 expression level was negatively correlated with serum urea levels (r = − 0.515, p = 0.024) and TID score (r = − 0.667, p = 0.007). Serum urea levels were positively correlated with Cr (r = 0.760, p < 0.001) and TID score (r = 0.672, p = 0.002) (Fig. 3D, E). Also, serum Cr levels were positively correlated with TID score (r = 0.641, p = 0.004). No other significant correlation was found between the evaluated parameters.
The correlations among SIRT1, urea, creatinine (Cr), and tubulointerstitial damage (TID) score. A Renal SIRT1 expression levels were negatively correlated with serum urea levels (r = − 0.515, p = 0.024). B The negative correlation between renal SIRT1 expression and Cr levels was not significant (r = − 0.423, p = 0.071). C Renal SIRT1 expression levels were negatively correlated with TID score (r = − 0.667, p = 0.007). D Serum urea levels were positively correlated with Cr levels (r = 0.760, p < 0.001). E Serum urea levels were positively correlated with TID score (r = 0.672, p = 0.002). F Serum Cr levels were positively correlated with TID score (r = 0.641, p = 0.004)
Discussion
In the present study, the effects of REM and DEX on rat kidney structure and function were evaluated with a particular focus on probable renal SIRT1 expression alteration.
REM, an antiviral medicine, and DEX, a glucocorticoid with anti-inflammatory effects, both have been used to treat COVID-19 [5, 6]. After administration, REM is converted to an active triphosphate metabolite which then is used as a substrate for viral RNA-dependent RNA synthetase and eventually terminates viral RNA transcription [16]. REM and DEX have been shown to improve COVID-19-related clinical outcomes and reduce its mortality risk or recovery time [5, 6].
In a study by Benfield et al., the effectiveness of standard of care (SOC) alone was compared with SOC plus REM and DEX in hospitalized patients with COVID-19. Treatment of moderate to severe COVID-19 in the group receiving REM and DEX was associated with reduced 30-day mortality and the need for mechanical ventilation compared to the individuals receiving SOC alone.
Nevertheless, these drugs may accompany some adverse effects [8, 17, 18]. In the present study, the TID score and serum urea levels in REM + DEX-administered rats were significantly higher than those in the control and DEX groups. Also, the mean serum Cr levels were significantly higher in the REM and REM + DEX groups compared to the control group. These results could indicate that the kidney injury and elevated TID score and urea and Cr levels in the rats might be predominantly due to REM administration than DEX. Acute kidney injury (AKI) has been frequently reported in REM-administered COVID-19 patients [17, 18]. In a retrospective case–control study by Chouchana et al. [18], among 5532 studied COVID-19 patients, 434 (7.8%) cases had kidney disorder and 327 (75.35%) of whom were receiving REM. No other medicine was prescribed to the majority of the cases [316 (96.6%)] at the onset of renal impairment. Of the 327 REM-receiving patients, 301 cases had severe conditions, and 15 (4.6%) had fatal outcomes. However, some studies reported conflicting results. Xu et al. [16] reported that REM could ameliorate renal fibrosis in obstructed kidneys. This effect was correlated with reduced SMAD3 phosphorylation and SMAD7 upregulation in vitro and in vivo. In their study, renal function–related markers, urea and Cr, were unchanged in REM-administered unilateral ureteral obstruction (UUO) mice. It is noteworthy that UUO is a classic model of renal fibrosis study; therefore, it might not be suitable to evaluate pharmaceutical effects on renal function. The differences in the applied study conditions and in vitro and in vivo models might cause these conflicting results.
Although the underlying mechanisms of REM-caused renal injury have not been completely understood, in the present study, renal SIRT1 expression was significantly lower in the REM + DEX-received rats than in the controls. The SIRT1 gene is located on chromosome 10q22.1 and contains nine exons and eight introns. The gene product, a class III histone deacetylase, is composed of about 500 amino acid residues. It is expressed in several organs such as the kidneys, liver, brain, and adipose tissues. In the kidneys, SIRT1 has been found predominantly in medullary tubular cells and moderately in cortical proximal tubular cells. It exerts renoprotective effects by reducing interstitial fibrosis, enhancing resistance to renal stresses, and inhibiting tubular and glomerular cell apoptosis and inflammation [12]. These effects are mediated by employing several substrates and pathways such as H1/H3/H4 histones, protein 53 (p53), p38, p65, transforming growth factor beta 1 (TGF-β1), SMADs, extracellular signal‑regulated protein kinases (ERK1/2), matrix metalloproteinase 14 (MMP-14), forkhead-box transcription factors (FOXOs), peroxisome proliferator-activated receptor coactivator-1α (PGC-1α), nuclear factor- κB (NF-κB), hypoxia-inducible factor (HIF)-2α, Ku70, Claudin-1, and A disintegrin and metalloproteinase domain 17 (ADAM17) [12, 19,20,21]. In the present study, the renal SIRT1 expression level was negatively correlated with serum urea levels and TID score. It has been revealed that SIRT1 downregulates ADAM17, a proteinase encoding gene. This proteinase enhances releasing of pro-inflammatory mediators such as tumor necrosis factor (TNF-α), interleukin 1β (IL-1β), and interleukin 6 (IL-6). Therefore, SIRT1 exerts anti-inflammatory effects by downregulating ADAM17. Downregulation of SIRT1 causes uncontrolled increases in pro-inflammatory cytokines and inflammation that eventually could lead to kidney injury [21]. Taken together, the nephrotoxicity induced by the administered drugs in the present study might be exacerbated by the SIRT1 downregulation.
In conclusion, REM and DEX have been commonly prescribed to patients with COVID-19. Although these drugs have been revealed to improve COVID-19-related clinical outcomes and reduce mortality risk or recovery time, these may be accompanied with adverse effects, including nephrotoxicity. The present study showed that the administration of DEX and mainly REM could lead to kidney injury which might be exacerbated or mediated by SIRT1 downregulation. Renal protein expression of SIRT1 was not evaluated in the present study which could be considered a limitation of our study. Further investigations in the COVID-infected animals, with different dosages of the drugs and over several times, are necessary to confirm our results. Other theoretical mechanisms for REM-induced nephrotoxicity and those related factors are imperative to evaluate in the future studies. The possible effects of other antiviral drugs and formulations on SIRT1 expression and their probable adverse effects could also be evaluated and compared with REM in the next studies.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
References
Tarmahi, V., Afghan, M., Raeisi, S., YousefiNodeh, H. R., Tamaddon, M., Moahamadzadeh, F., Mohammadzadeh, N., & Ebadi, Z. (2021). The accuracy of enzyme-linked immunosorbent assay in the diagnosis of COVID-19 in Iranian children. International Journal of Pediatrics, 9(7), 13937–13945.
Rabby, M. I. I. (2020). Current drugs with potential for treatment of COVID-19: A literature review: Drugs for the treatment process of COVID-19. Journal of Pharmacy & Pharmaceutical Sciences, 23, 58–64.
Abd-Elsalam, S., Salama, M., Soliman, S., Naguib, A. M., Ibrahim, I. S., Torky, M., Abd El Ghafar, M. S., Abdul-Baki, E.A.-R.M., & Elhendawy, M. (2022). Remdesivir efficacy in COVID-19 treatment: A randomized controlled trial. The American Journal of Tropical Medicine and Hygiene, 106(3), 886.
de Oliveira Silva, N. A., de SeneAmâncio Zara, A. L., Figueras, A., & de Melo, D. O. (2021). Potential kidney damage associated with the use of remdesivir for COVID-19: Analysis of a pharmacovigilance database. Cadernos Saude Publica, 37, e00077721.
Marrone, A., Nevola, R., Sellitto, A., Cozzolino, D., Romano, C., Cuomo, G., Aprea, C., Schwartzbaum M. X. P., Ricozzi C., Imbriani. S. (2022). Remdesivir plus dexamethasone versus dexamethasone alone for the treatment of COVID-19 patients requiring supplemental O2 therapy: A prospective controlled non-randomized study. Clinical Infectious Diseases: an Official Publication of the Infectious Diseases Society of America, 75(1):e403–e409
Benfield, T., Bodilsen, J., Brieghel, C., Harboe, Z. B., Helleberg, M., Holm, C., Israelsen, S. B., Jensen, J., Jensen, T. Ø., & Johansen, I. S. (2021). Improved survival among hospitalized patients with coronavirus disease 2019 (COVID-19) treated with remdesivir and dexamethasone. A nationwide population-based cohort study. Clinical Infectious Diseases, 73(11), 2031–2036.
Rahimi, M. M., Jahantabi, E., Lotfi, B., Forouzesh, M., Valizadeh, R., & Farshid, S. (2021). Renal and liver injury following the treatment of COVID-19 by remdesivir. Journal of Nephropathology, 10(2), 1–4.
Chen, F., Hao, L., Zhu, S., Yang, X., Shi, W., Zheng, K., Wang, T., & Chen, H. (2021). Potential adverse effects of dexamethasone therapy on COVID-19 patients: Review and recommendations. Infectious Diseases and Therapy, 10(4), 1907–1931.
Singh, A., & Kamath, A. (2021). Assessment of adverse events associated with remdesivir use for coronavirus disease 2019 using real-world data. Expert Opinion on Drug Safety, 20(12), 1559–1564.
Yin, L., Zhao, H., Zhang, H., Li, Y., Dong, Y., Ju, H., Kong, F., & Zhao, S. (2021). Remdesivir alleviates acute kidney injury by inhibiting the activation of NLRP3 inflammasome. Frontiers in Immunology, 12, 652446.
Bazyluk, A., Malyszko, J., Hryszko, T., & Zbroch, E. (2019). State of the art–sirtuin 1 in kidney pathology–clinical relevance. Advances in Medical Sciences, 64(2), 356–364.
Dong, Y.-j., Liu, N., Xiao, Z., Sun T., Wu, S.-h., Sun, W.-x., Xu, Z.-g., Yuan H. (2014). Renal protective effect of sirtuin 1. Journal of Diabetes Research, D843786:8
Goldman, J. D., Lye, D. C., Hui, D. S., Marks, K. M., Bruno, R., Montejano, R., Spinner, C. D., Galli, M., Ahn, M.-Y., & Nahass, R. G. (2020). Remdesivir for 5 or 10 days in patients with severe COVID-19. New England Journal of Medicine, 383(19), 1827–1837.
Hummon, A. B., Lim, S. R., Difilippantonio, M. J., & Ried, T. (2007). Isolation and solubilization of proteins after TRIzol® extraction of RNA and DNA from patient material following prolonged storage. BioTechniques, 42(4), 467–472.
Park, J. W., Bae, E. H., Kim, I. J., Ma, S. K., Choi, C., Lee, J., & Kim, S. W. (2010). Paricalcitol attenuates cyclosporine-induced kidney injury in rats. Kidney international, 77(12), 1076–1085.
Xu, L., Tan, B., Huang, D., Yuan, M., Li, T., Wu, M., & Ye, C. (2021). Remdesivir inhibits tubulointerstitial fibrosis in obstructed kidneys. Frontiers in Pharmacology, 12, 1668.
Dubert, M., Visseaux, B., Isernia, V., Bouadma, L., Deconinck, L., Patrier, J., Wicky, P.-H., Le Pluart, D., Kramer, L., & Rioux, C. (2020). Case report study of the first five COVID-19 patients treated with remdesivir in France. International Journal of Infectious Diseases, 98, 290–293.
Chouchana, L., Preta, L.-H., Tisseyre, M., Terrier, B., Treluyer, J.-M., & Montastruc, F. (2021). Kidney disorders as serious adverse drug reactions of remdesivir in coronavirus disease 2019: A retrospective case–noncase study. Kidney International, 99(5), 1235–1236.
Li, P., Liu, Y., Qin, X., Chen, K., Wang, R., Yuan, L., Chen, X., Hao, C., & Huang, X. (2021). SIRT1 attenuates renal fibrosis by repressing HIF-2α. Cell Death Discovery, 7(1), 1–10.
Ren, H., Shao, Y., Wu, C., Ma, X., Lv, C., & Wang, Q. (2020). Metformin alleviates oxidative stress and enhances autophagy in diabetic kidney disease via AMPK/SIRT1-FoxO1 pathway. Molecular and Cellular Endocrinology, 500, 110628.
Miller, R., Wentzel, A., & Richards, G. (2020). COVID-19: NAD+ deficiency may predispose the aged, obese and type2 diabetics to mortality through its effect on SIRT1 activity. Medical Hypotheses, 144, 110044.
Acknowledgements
This work was supported by Biotechnology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: MMA, NR, and AG. Performed the experiments: SD, SR, HA, DS, LR, HP, SA, and JM. Analyzed the data: MB. Contributed reagents/materials/analysis tools: MMA, and AG. The first draft of the manuscript was written by SD and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval
The protocol was approved by the research ethics committee of Tabriz University of Medical Sciences (IR.TBZMED.VCR.REC.1400.417).
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Danaiyan, S., Abbasi, M.M., Raeisi, S. et al. The Effects of Remdesivir and Dexamethasone on Renal Sirtuin-1 Expression and Renal Function in Male Rats. Appl Biochem Biotechnol 196, 632–642 (2024). https://doi.org/10.1007/s12010-023-04529-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04529-3