Abstract
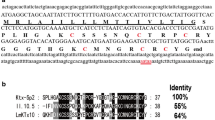
Snake venoms are a potential source of bioactive peptides, which have multiple therapeutic properties in treating diseases such as diabetes, cancer, and neurological disorders. Among bioactive peptides, cytotoxins (CTXs) and neurotoxins are low molecular weight proteins belonging to the three-finger-fold toxins (3FTxs) family composed of two β sheets that are stabilized by four to five conserved disulfide bonds containing 58–72 amino acid residues. These are highly abundant in snake venom and are predicted to have insulinotropic activities. In this study, the CTXs were purified from Indian cobra snake venom using preparative HPLC and characterized using high-resolution mass spectrometry (HRMS) TOF–MS/MS. Further SDS-PAGE analysis confirmed the presence of low molecular weight cytotoxic proteins. The CTXs in fractions A and B exhibited dose-dependent insulinotropic activity from 0.001 to 10 µM using rat pancreatic beta-cell lines (RIN-5F) in the ELISA. Nateglinide and repaglinide are synthetic small-molecule drugs that control sugar levels in the blood in type 2 diabetes, which were used as a positive control in ELISA. Concluded that purified CTXs have insulinotropic activity, and there is a scope to use these proteins as small molecules to stimulate insulinotropic activities. At this stage, the focus is on the efficiency of the cytotoxins to induce insulin. Additional work is ongoing on animal models to see the extent of the beneficial effects and efficiency to cure diabetes using streptozotocin-induced models.












Similar content being viewed by others
Data Availability
All datasets generated for this study are included in the article/Supplementary Material.
References
Hu, F. B. (2011). Globalization of diabetes: The role of diet, lifestyle, and genes. Diabetes Care, 34(6), 1249–1257.
Saeedi, P., Petersohn, I., Salpea, P., Malanda, B., Karuranga, S., Unwin, N., Colagiuri, S., Guariguata, L., Motala, A. A., Ogurtsova, K., & Shaw, J. E. (2019). Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Research and Clinical Practice, 157, 107843.
Cade, W. T. (2008). Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Physical Therapy, 88(11), 1322–1335.
Cerf, M. E. (2013). Beta cell dysfunction and insulin resistance. Frontiers in Endocrinology (Lausanne), 4, 37. https://doi.org/10.3389/fendo.2013.00037
Akbarian, M., Khani, A., Eghbalpour, S., & Uversky, V. N. (2022). Bioactive peptides: Synthesis, sources, applications, and proposed mechanisms of action. International Journal of Molecular Sciences, 23(3), 1445.
Sarmiento, B. E., Santos Menezes, L. F., & Schwartz, E. F. (2019). Insulin release mechanism modulated by toxins isolated from animal venoms: From basic research to drug development prospects. Molecules, 24(10), 1846.
de la Torre, B. G., & Albericio, F. (2020). Peptide therapeutics 2.0. Molecules, 25(10), 2293.
Wang, R., McGrath, B. C., Kopp, R. F., Roe, M. W., Tang, X., Chen, G., & Cavener, D. R. (2013). Insulin secretion and Ca2+ dynamics in β-cells are regulated by PERK (EIF2AK3) in concert with calcineurin. Journal of Biological Chemistry, 288(47), 33824–33836.
Nguyen, T. T. N., Folch, B., Létourneau, M., Vaudry, D., Truong, N. H., Doucet, N., Chatenet, D., & Fournier, A. (2012). Cardiotoxin-I: An unexpectedly potent insulinotropic agent. ChemBioChem, 13(12), 1805–1812.
Munawar, A., Ali, S. A., Akrem, A., & Betzel, C. (2018). Snake venom peptides: Tools of biodiscovery. Toxins, 10(11), 474.
Li, S., Wang, J., Zhang, X., Ren, Y., Wang, N., Zhao, K., Chen, X., Zhao, C., Li, X., Shao, J., Yin, J., West, M. B., Xu, N., & Liu, S. (2004). Proteomic characterization of two snake venoms: Naja naja atra and Agkistrodon halys. Biochemical Journal, 384(1), 119–127.
Ständker, L., Harvey, A. L., Fürst, S., Mathes, I., Forssmann, W. G., de Motta, G. E., & Beress, L. (2012). Improved method for the isolation, characterization and examination of neuromuscular and toxic properties of selected polypeptide fractions from the crude venom of the Taiwan cobra Naja naja atra. Toxicon, 60(4), 623–631.
Quintana-Castillo, J. C., Vargas, L. J., Segura, C., Estrada-Gómez, S., Bueno-Sánchez, J. C., & Alarcón, J. C. (2018). Characterization of the venom of C. d. cumanesis of Colombia: Proteomic analysis and antivenomic study. Toxins, 10(2), 85.
Slagboom, J., Kaal, C., Arrahman, A., Vonk, F. J., Somsen, G. W., Calvete, J. J., Wüster, W., & Kool, J. (2022). Analytical strategies in venomics. Microchemical Journal, 175, 107187.
El-Aziz, T. M. A., Jaquillard, L., Bourgoin-Voillard, S., Martinez, G., Triquigneaux, M., Zoukimian, C., Combemale, S., Hograindleur, J. P., al Khoury, S., Escoffier, J., Michelland, S., Bulet, P., Beroud, R., Seve, M., Arnoult, C., & de Waard, M. (2020). Identification, characterization and synthesis of walterospermin, a sperm motility activator from the egyptian black snake walterinnesia aegyptia venom. International Journal of Molecular Sciences, 21(20), 1–25.
Vejayan, J., Shin Yee, L., Ponnudurai, G., Ambu, S., & Ibrahim, I. (2010). Protein profile analysis of Malaysian snake venoms by two-dimensional gel electrophoresis. Journal of Venomous Animals and Toxins including Tropical Diseases, 16, 623–630.
Gerlier, D., & Thomasset, N. (1986). Use of MTT colorimetric assay to measure cell activation. Journal of Immunological Methods, 94(1–2), 57–63.
Bordoli, L., Kiefer, F., Arnold, K., Benkert, P., Battey, J., & Schwede, T. (2009). Protein structure homology modeling using SWISS-MODEL workspace. Nature Protocols, 4(1), 1–13.
Waterhouse, A., Bertoni, M., Bienert, S., Studer, G., Tauriello, G., Gumienny, R., Heer, F. T., de Beer, T. A. P., Rempfer, C., Bordoli, L., & Lepore, R. (2018). SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Research, 46(W1), W296–W303.
Schwede, T., Kopp, J., Guex, N., & Peitsch, M. C. (2003). SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Research, 31(13), 3381–3385.
Kim, S., Thiessen, P. A., Bolton, E. E., Chen, J., Fu, G., Gindulyte, A., Han, L., He, J., He, S., Shoemaker, B. A., & Wang, J. (2016). PubChem substance and compound databases. Nucleic Acids Research, 44(D1), D1202–D1213.
Wang, Y., Xiao, J., Suzek, T. O., Zhang, J., Wang, J., & Bryant, S. H. (2009). PubChem: a public information system for analyzing bioactivities of small molecules. Nucleic Acids Research, 37(suppl_2), W623–W633.
Ali, A., Kuo, W. W., Kuo, C. H., Lo, J. F., Chen, M. Y., Daddam, J. R., Ho, T. J., Viswanadha, V. P., Shibu, M. A., & Huang, C. Y. (2021). E3 ligase activity of carboxyl terminus of Hsc70 interacting protein (CHIP) in Wharton’s jelly derived mesenchymal stem cells improves their persistence under hyperglycemic stress and promotes the prophylactic effects against diabetic cardiac damages. Bioengineering & Translational Medicine, 6(3), e10234.
Raj, I., Kumar, S., & Gourinath, S. (2012). The narrow active-site cleft of O-acetylserine sulfhydrylase from Leishmania donovani allows complex formation with serine acetyltransferases with a range of C-terminal sequences. Acta Crystallographica Section D: Biological Crystallography, 68(8), 909–919.
Meetei, P. A., Rathore, R. S., Prabhu, N. P., & Vindal, V. (2016). Modeling of babesipain-1 and identification of natural and synthetic leads for bovine babesiosis drug development. Journal of Molecular Modeling, 22(4), 1–14.
Churchill, C. D., Klobukowski, M., & Tuszynski, J. A. (2016). Analysis of the binding mode of laulimalide to microtubules: Establishing a laulimalide–tubulin pharmacophore. Journal of Biomolecular Structure and Dynamics, 34(7), 1455–1469.
Dallakyan, S., & Olson, A. J. (2015). Small-molecule library screening by docking with PyRx. Chemical biology (pp. 243–250). New York, NY: Humana Press.
Gnanaraj, C., Sekar, M., Fuloria, S., Swain, S. S., Gan, S. H., Chidambaram, K., Rani, N. N. I. M., Balan, T., Stephenie, S., Lum, P. T., & Jeyabalan, S. (2022). In silico molecular docking analysis of karanjin against alzheimer’s and parkinson’s diseases as a potential natural lead molecule for new drug design, development and therapy. Molecules, 27(9), 2834.
Trott, O., & Olson, A. J. (2010). AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. Journal of Computational Chemistry, 31(2), 455–461.
Lewis, R. J., & Garcia, M. L. (2003). Therapeutic potential of venom peptides. Nature Reviews Drug Discovery, 2(10), 790–802.
Acknowledgements
This work is partly supported by the Kyntox Biotech India Pvt Ltd, Bangalore, Bangalore Bio-innovation Center, SCIEX, India and BioNome, Bangalore.
Author information
Authors and Affiliations
Contributions
Upendra Gunta: Data curation; Formal analysis; Conceptualization; Investigation; Validation; Writing – original draft, reviewing and editing the manuscript. Gangadhar P Vadla: Formal analysis; Conceptualization; Investigation; Supervision, review and editing. Gopi Kadiyala: Formal analysis; Review. Dilipkumar Reddy Kandula: Formal analysis; Review and editing. M. Mastan: Methodology; Conceptualization; Investigation; Supervision, Validation; Writing–original draft; Writing–review and editing.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 1.53 mb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gunta, U., Vadla, G.P., Kadiyala, G. et al. Identification of Potential Insulinotropic Cytotoxins from Indian Cobra Snake Venom Using High-Resolution Mass Spectrometry and Analyzing Their Possible Interactions with Potassium Channel Receptors by In Silico Studies. Appl Biochem Biotechnol 196, 160–181 (2024). https://doi.org/10.1007/s12010-023-04523-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04523-9