Abstract
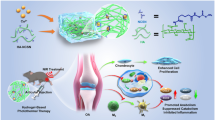
Articular cartilage defect treatment is a very important problem because its therapeutic options are not successful enough. Due to the weak self-repairing capacity of the avascular cartilage, even minor damage can progress and cause joint damage leading to osteoarthritis. Although various treatment strategies have been developed to repair damaged cartilage, cell- and exosome-based therapies are promising. Plant extracts have been used for decades, and their effects on cartilage regeneration have been studied. Exosome-like vesicles, which are secreted by all living cells, are involved in cell-to-cell communication and cell homeostasis. The differentiation potential of exosome-like vesicles isolated from S. lycopersicum and C. limon, which are known to have anti-inflammatory and antioxidant properties, was investigated in the differentiation of human adipose-derived mesenchymal stem cells (hASCs) into chondrocytes. In order to obtain tomato-derived exosome-like vesicles (TELVs) and lemon-derived exosome-like vesicles (LELVs) Aquous Two- Phase system was performed. Characterisation of isolated vesicles based on size, shape were achived via Zetasizer, NTA FAME analysis, and SEM techniques. These results showed that TELVs and LELVs increased cell viability and did not show any toxic effects on stem cells. Although TELVs triggered chondrocyte formation, LELVs downregulated. The expression of ACAN, SOX9, and COMP, known as chondrocyte markers, was increased by TELV treatment. In addition, protein expression of the two most important proteins, COL2 and COLXI, found in the extracellular matrix of cartilage, increased. These findings suggest that TELVs can be used for cartilage regeneration, and may be a novel and promising treatment for osteoarthritis.






Similar content being viewed by others
Data Availability
Data available on request from the authors.
References
Casanova, M. R., Osório, H., Reis, R. L., Martins, A., & Neves, N. M. (2021). Chondrogenic differentiation induced by extracellular vesicles bound to a nanofibrous substrate. NPJ Regenerative medicine, 6(1), 1–12.
Mao, A. S., & Mooney, D. J. (2015). Regenerative medicine: Current therapies and future directions. Proceedings of the National Academy of Sciences, 112(47), 14452–14459.
Choi, S., Kim, G. M., Maeng, Y. H., Kang, H., Teong, C. T., Lee, E. E., & Kim, M. K. (2018). Autologous bone marrow cell stimulation and allogenic chondrocyte implantation for the repair of full-thickness articular cartilage defects in a rabbit model. Cartilage, 9(4), 402–409.
Hosseinzadeh, M., Kamali, A., Hosseini, S., & Baghaban Eslaminejad, M. (2021). Higher chondrogenic potential of extracellular vesicles derived from mesenchymal stem cells compared to chondrocytes-EVs in vitro. BioMed Research International
Nasiri, N., Hosseini, S., Alini, M., Khademhosseini, A., & Eslaminejad, M. B. (2019). Targeted cell delivery for articular cartilage regeneration and osteoarthritis treatment. Drug Discovery Today, 24(11), 2212–2224.
Ochs, B. G., Mueller-Horvat, C., Albrecht, D., Schewe, B., Weise, K., Aicher, W. K., & Rolauffs, B. (2011). Remodeling of articular cartilage and subchondral bone after bone grafting and matrix-associated autologous chondrocyte implantation for osteochondritis dissecans of the knee. The American Journal of Sports Medicine, 39(4), 764–773.
Ni, Z., Kuang, L., Chen, H., Xie, Y., Zhang, B., Ouyang, J., & Chen, L. (2019). The exosome-like vesicles from osteoarthritic chondrocyte enhanced mature IL-1β production of macrophages and aggravated synovitis in osteoarthritis. Cell Death & Disease, 10(7), 1–16.
Hill, C. L., Hunter, D. J., Niu, J., Clancy, M., Guermazi, A., Genant, H., & Felson, D. T. (2007). Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Annals of the Rheumatic Diseases, 66(12), 1599–1603.
Robinson, W. H., Lepus, C. M., Wang, Q., Raghu, H., Mao, R., Lindstrom, T. M., & Sokolove, J. (2016). Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nature Reviews Rheumatology, 12(10), 580–592.
To, K., Romain, K., Mak, C., Kamaraj, A., Henson, F., & Khan, W. (2020). The treatment of cartilage damage using human mesenchymal stem cell-derived extracellular vesicles: A systematic review of in vivo studies. Frontiers in Bioengineering and Biotechnology, 8, 580.
Davies-Tuck, M. L., Wluka, A. E., Wang, Y., Teichtahl, A. J., Jones, G., Ding, C., & Cicuttini, F. M. (2008). The natural history of cartilage defects in people with knee osteoarthritis. Osteoarthritis and Cartilage, 16(3), 337–342.
Mao, G., Xu, Y., Long, D., Sun, H., Li, H., Xin, R., ... & Kang, Y. (2021). Exosome-transported circRNA_0001236 enhances chondrogenesis and suppress cartilage degradation via the miR-3677–3p/Sox9 axis. Stem Cell Research & Therapy, 12(1), 1–14.
Martel-Pelletier, J., Barr, A. J., Cicuttini, F. M., Conaghan, P. G., Cooper, C., Goldring, M. B., Goldring, S. R., Jones, G., Teichtahl, A. J., & Pelletier, J. P. (2016). Osteoarthritis. Nature reviews. Disease primers, 2, 16072.
Bosnakovski, D., Mizuno, M., Kim, G., Takagi, S., Okumura, M., & Fujinaga, T. (2006). Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: Influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnology and Bioengineering, 93(6), 1152–1163.
Feng, D., Zhao, W. L., Ye, Y. Y., Bai, X. C., Liu, R. Q., Chang, L. F., ... & Sui, S. F. (2010). Cellular internalization of exosomes occurs through phagocytosis. Traffic, 11(5), 675–687.
Valadi, H., Ekström, K., Bossios, A., Sjöstrand, M., Lee, J. J., & Lötvall, J. O. (2007). Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature Cell Biology, 9(6), 654–659.
Van der Pol, E., Böing, A. N., Harrison, P., Sturk, A., & Nieuwland, R. (2012). Classification, functions, and clinical relevance of extracellular vesicles. Pharmacological Reviews, 64(3), 676–705.
Woo, C. H., Kim, H. K., Jung, G. Y., Jung, Y. J., Lee, K. S., Yun, Y. E., & Cho, Y. W. (2020). Small extracellular vesicles from human adipose-derived stem cells attenuate cartilage degeneration. Journal of Extracellular Vesicles, 9(1), 1735249.
Alenquer, M., & Amorim, M. J. (2015). Exosome biogenesis, regulation, and function in viral infection. Viruses, 7(9), 5066–5083.
Meyer, D., Pajonk, S., Micali, C., O’Connell, R., & Schulze-Lefert, P. (2009). Extracellular transport and integration of plant secretory proteins into pathogen-induced cell wall compartments. The Plant Journal, 57(6), 986–999.
Sumalan, R. M., Ciulca, S. I., Poiana, M. A., Moigradean, D., Radulov, I., Negrea, M., ... & Sumalan, R. L. (2020). The antioxidant profile evaluation of some tomato landraces with soil salinity tolerance correlated with high nutraceuticaland functional value. Agronomy, 10(4), 500.
Tam, L. S., Gu, J., & Yu, D. (2010). Pathogenesis of ankylosing spondylitis. Nature Reviews Rheumatology, 6(7), 399–405.
Lim, C. T., Lolli, F., Thomas, J. D., Kola, B., & Korbonits, M. (2012). Measurement of AMP-activated protein kinase activity and expression in response to ghrelin. In Methods in Enzymology (Vol. 514 271–287). Academic Press.
Brown, S. C., Palazuelos, M., Sharma, P., Powers, K. W., Roberts, S. M., Grobmyer, S. R., & Moudgil, B. M. (2010). Nanoparticle characterization for cancer nanotechnology and other biological applications. Methods in Molecular Biology, 624, 39–65.
Sokolova, V., Ludwig, A.-K., Hornung, S., Rotan, O., Horn, P. A., Epple, M., et al. (2011). Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy. Colloids and Surfaces. B, Biointerfaces, 87(1), 146–150.
Ju, S., Mu, J., Dokland, T., Zhuang, X., Wang, Q., Jiang, H., ... & Zhang, H. G. (2013). Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Molecular Therapy, 21(7), 1345–1357.
Kabakov, A. E., & Gabai, V. L. (2018). “Cell death and survival assays. Methods in molecular biology” (Clifton NJ), 1709,107–127.
Robert, S., Lacroix, R., Poncelet, P., Harhouri, K., Bouriche, T., Judicone, C., ... & Dignat-George, F. (2012). High-sensitivity flow cytometry provides access to standardized measurement of small-size microparticles—brief report. Arteriosclerosis, Thrombosis, and Vascular Biology, 32(4), 1054–1058.
Wu, Z., Sun, S., Wang, F., & Guo, D. (2011). Establishment of regeneration and transformation system of Lycopersicon esculentum MicroTom. British Biotechnology Journal, 1(3), 53–60.
Raiola, A., Rigano, M. M., Calafiore, R., Frusciante, L., & Barone, A. (2014). Enhancing the health-promoting effects of tomato fruit for biofortified food. Mediators of inflammation.
Doyle, L. M., & Wang, M. Z. (2019). Cells, 8, 727.
Özkan, İ, Koçak, P., Yıldırım, M., Ünsal, N., Yılmaz, H., Telci, D., & Şahin, F. (2021). Garlic (Allium sativum)-derived SEVs inhibit cancer cell proliferation and induce caspase mediated apoptosis. Scientific Reports, 11(1), 1–11.
Sekiya, I., Tsuji, K., Koopman, P., Watanabe, H., Yamada, Y., Shinomiya, K., & Noda, M. (2000). SOX9 enhances aggrecan gene promoter/enhancer activity and is up-regulated by retinoic acid in a cartilage-derived cell line, TC6. Journal of Biological Chemistry, 275(15), 10738–10744.
Watanabe, H., Yamada, Y., & Kimata, K. (1998). Roles of aggrecan, a large chondroitin sulfate proteoglycan, in cartilage structure and function. The Journal of Biochemistry, 124(4), 687–693.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Merve Yıldırım, Bilge Kabataş, and Naz Ünsal. Olcay Eren helped with data analysis and manuscript writing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
This study was supported by Yeditepe University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yıldırım, M., Ünsal, N., Kabataş, B. et al. Effect of Solanum lycopersicum and Citrus limon–Derived Exosome-Like Vesicles on Chondrogenic Differentiation of Adipose-Derived Stem Cells. Appl Biochem Biotechnol 196, 203–219 (2024). https://doi.org/10.1007/s12010-023-04491-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04491-0