Abstract
Postweaning stress in mammalian in vivo models leads to significant oxidative stress in the body as well as inducing hormonal disturbance. In this study, we assessed progressive alterations in reactive oxygen species (ROS), which at high levels can show oxidative stress, in addition to oxidative damage to the DNA structure of rabbits. Different groups of rabbits were fasted for 48 h per week for 3 weeks, fed a commercial diet with probiotics added (200 mg of Bacillus licheniformis and Bacillus subtilis), and fasted while being treated with probiotics. The results showed that weaning induced a significant elevation in oxidative stress markers, such as the ROS-related genes malate dehydrogenase 1 (MDH1) and flavin-containing monooxygenase 2 (FMO2), DNA damage, and hormonal disturbance. However, probiotic treatment resulted in significant decreases in the levels of malondialdehyde, cortisol, and triiodothyronine (T3); DNA damage; and apoptosis, as well as changes in the expression of ROS-related genes. On the other hand, supplementation with probiotics reduced these postweaning stress signs in fasted animal models by elevating the genes encoding catalase and superoxide dismutase as well as increasing glutathione peroxidase (GSH-Px), glutathione–s-transferase, alkaline phosphatase, glucose, and thyroxin (T4) levels. The results suggest that supplementation with probiotics accompanied by a fasting program could decrease oxidative stress, ROS genes, and genomic DNA damage and improve the hormonal status that is induced by postweaning stress in mammalian in vivo models.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The digestive process of the rabbit is highly sophisticated and sensitive. This explains the rabbit’s sensitivity to enteric disorders, especially with exposure to stress. During aning, the diet of the rabbit is changed from milk to solid food. This sudden change can increase the rabbit’s vulnerability to digestive tract problems and microbial diseases [1]. Moreover, for the young mammal, the weaning and postweaning periods are particularly important for growth and feeding efficiency [2, 3]. Fasting in growing rabbits can have advantages, such as increased digestive efficiency, modification of the partition of body energy retention to protein instead of fat, and reduction of mortality and morbidity due to digestive problems [4]. Moreover, the amount and duration of restricted feeding is involved in the regulatory mechanisms of metabolism in animals. Restricted feeding may positively affect several changes in metabolic disorders that lead to hormonal changes, immune depression, and altered digestive system functions, especially in the liver and small intestine [3]. Furthermore, fasting can quickly retrieve the morphology and functions of the intestine [5]. Increase in the endogenous production of adrenal corticoids from the adrenal glands following the action of stress factors such as fasting has been demonstrated [6]. On the other hand, a long period of nutrient reduction during development results in homeostatic reactions of the corticotropic, somatotropic, and thyrotropic axes [7]. In addition, weaning leads to significant oxidative stress and apoptosis in the body as well as inducing reactive oxygen species (ROS)-related genes and hormonal disturbances [8, 9]. Preliminary evidence suggests that probiotics have a potent therapeutic advantage in this condition [10, 11].
It is known that DNA is the main target of oxidative stress on mammalian cells. Many studies have shown that the pathophysiology of many diseases, such as diabetes, atherosclerosis, cancer, and neurological diseases, is caused by persistent oxidative damage to DNA [12]. Oxidative damage to the genetic materials in the genome depends mainly on the susceptibility to oxidative attack on the nucleotide sequences of the genetic material, and the ability for successful repair processes. Studies have shown that one of the most vulnerable DNA nucleotide sequences to attack and oxidative damage are the non-coding sequences due to its basic structure [13]. It was found that the most nitrogenous bases of DNA for oxidative modification are guanine compared to the other three nitrogenous bases on the genetic material. As a result of the oxidative attack of nitrogenous bases, 8-Hydroxyguanine (8-OH-Gua) is formed as one of the common marker of oxidant-induced DNA damage [14].
Probiotics are living microorganisms like; yeast, active bacteria, or bacterial spores’ possess the ability to prevent enteric diseases in rabbits. Probiotics encourage gut colonization as well as stabilizing eubiosis by competing with the growth of harmful microorganisms and enhancing digestive enzymes and vitamin secretion by lowering the intestinal pH. Probiotic preparations are added to the diet to enhance fermentation in the digestive system. Probiotics include Bacillus licheniformis and Bacillus subtilis. Probiotic spores have the ability to persist in the bacterial pelletization process [15] and passage through the stomach. Probiotic spores can sprout in the rabbit’s intestines and consume carbohydrates in huge amounts for their growth, in addition to manufacturing digestive enzymes such as amylase, protease, and lipase. These enzymes catalyze and improve the digestion of food. During weaning, the digestive system passes through an adaptation stage. In this stressful state, small rabbits are more sensitive to illnesses, and they grow slowly [16]. Therefore, administration of probiotics accompanied by a fasting regime could relieve weaning shock and alleviate oxidative stress in addition to reducing mortality and morbidity. We tested this hypothesis on recently weaned male white New Zealand rabbits.
Materials and Methods
Animal Management and Feeding
This study was performed at a rabbit farm of Animal physiology Lab, Faculty of Agriculture, Cairo University, Giza, Egypt. Twenty four recently weaned 5-week-old male white New Zealand rabbits (525 ± 8.34 g. BWT) were randomly distributed into four experimental groups (n = 6). The rabbits were housed in a naturally ventilated building and kept in individual wire galvanized cages. The rabbits were fed according to NRC [17] allowances on a concentrated feed mixture (crude protein 20%, crude fat 3%–4%, crude fiber 14%–15%, net energy 2640 kcal) mixed with Bersem Hegazy hay as roughage. Animals were offered their CFM once daily to measure feed consumption (FC) and feed conversion rate (FCR). The ingredients of the pellets are shown in Table 1.Water was available ad libitum, and the lighting program provided 16 h light and 8 h dark per day. This study was approved by the Institutional Animal Care and Use committee of Cairo University, protocol No. (CU-II-F37-17).
Experimental Design
The rabbits were allocated to four groups. Group 1: The rabbits were fed commercial diet ad libitum and served as a control group (Ad). Group 2: The rabbits fed the commercial diet ad libitum were fasted for 12 h/day twice a week (F12) for 3 weeks. Group 3: The rabbits were fed the commercial diet with 200 mg of prebiotics (Bacillus licheniformis and Bacillus subtilis) per liter of water for 3 weeks, (biofactor liquid). Group 4: The rabbits fed commercial diet were fasted for 48 h each week (F48) with 200 mg of prebiotic (Bacillus licheniformis and Bacillus subtilis) per liter of water for 3 weeks. During the last week of the experiment, all groups were fed ad libitum.
Growth Performance Traits
The animals’ body weight, daily feed intake, and the number of dead rabbits were recorded. The daily animal weight gain with its FCR was recorded per week, in addition to daily recording of the mortality rate during the experimental period (8 weeks).
Carcass Characteristics
By the end of the study, four rabbits (8 weeks of age) in each treatment group were chosen randomly, fasted for 12 h, weighed, and sacrificed. Then, the kidney, spleen, liver, and cecum were taken for molecular biological analysis.
Blood Samples for Determination of Oxidative Status Biomarkers
After the last day of experimental treatment, the animals were fasted overnight, and blood samples were collected. Blood plasma samples were separated into lithium heparin test tubes and centrifuged at 4000 rpm for 15 min; the plasma was then stored in a deep freezer at approximately − 20ºC until analysis. Total antioxidant capacity (TAC), total superoxide dismutase (T-SOD), catalase (CAT), and malondialdehyde (MDA) were analyzed as described by Koracevic et al. [18] and Richard et al. [19]. In addition, blood glutathione peroxidase (GPx) and glutathione–s-transferase (GST) activities were assessed according to Miranda et al. [20].
Hormone Assay
Blood plasma concentrations of thyroxin (T4) and triiodothyronine (T3) were measured according to Abdel-Fattah et al. [21] using the radioimmunoassay (RIA) technique. Blood plasma concentrations of cortisol were evaluated by RIA, using the CORT kit (ICN Biomedical Inc., Costa Mesa, CA, USA) according to Palme et al. [22].
Comet Assay
Comets were examined in liver samples according to Blasiak et al. [23]. Low-melting-point agarose was mixed with liver specimen (1:10 v/v). The mixture of the samples was loaded onto slides which were coated previously with normal agarose. The slides were kept in a dark place followed by covering it with low-melting-point agarose and left for 30 min at 4 °C. The slides were conserved in lysis solution for one hour followed by covering it in alkaline unwinding solution for another 1 h. After electrophoresis run the slides were washed in neutralizing buffer followed by immersing it in 70% ethanol. An ethidium bromide dye was used to stain the slides which were finally examined by a Zeiss epifluorescence microscope [24, 25].
Expression of Antioxidant- and ROS-Related Genes
Total RNA Isolation
Liver specimens were used to insulate the RNA of the rabbit’s tissues by TRIzol® Reagent (Invitrogen, Germany). The RNA molecules were confirmed in agarose gel (1.5%) stained with ethidium bromide to verify of the presence 28S and 18S bands. After assessment the purity of the RNA aliquots of the ribonucleic acids were prepared [26] for reverse transcription (RT).
RT Reaction
Segregated RNA from rabbit liver specimens was utilized to obtain cDNA through reverse transcription reaction using RevertAidTM First Strand cDNA Kit (Fermentas, Germany) containing oligo(dT)18 primer, dNTP Mix, Reverse Transcriptase enzyme and RNase Inhibitor. The reaction PCR products were used immediately for qRT-PCR or kept at − 20ºC up to use.
qRT-PCR Analysis
StepOne™ Real-Time PCR System (Applied Biosystems, USA) was used for amplification of the obtained cDNA using 1 × SYBR® Premix Ex TaqTM (TaKaRa, Biotech. Co. Ltd, China). The primer sequences of the specific genes tested (CAT, SOD1, MDH1, FMO2) designed using (Primer3 software) are presented in Table 2 [27]. The 2−ΔΔCT method was used to determine the quantity of the target.
Apoptosis Assay
Liver tissue samples were homogenized into single-cell suspensions by the method of Khalil et al. [28]. We detected apoptosis of cells by flow cytometry (FCM) assay using an Annexin V/propidium iodide (PI) apoptosis detection kit. The single-cell suspension (1 × 106 cells/mL) was put into 200 μL of ice-cold binding buffer, and 10 μL of horseradish peroxidase FITC-labeled Annexin V and 5 μL of PI was added. The cell suspension was incubated for 15 min in darkness at room temperature. The apoptosis rate was detected by flow cytometer as normal cells (FITC and PI-negative cells) and apoptotic or necrotic cells (FITC and PI-positive cells).
Statistical Analysis
The data were analyzed by the General Linear Models technique of the Statistical Analysis System (SAS, 1982), then by the Scheffé test to evaluate significant differences between groups. The results are presented as means ± SEM. All significance statements were based on P < 0.05.
Results and Discussion
The motive of this study was to assess the protective impact of probiotics administration accompanied by a fasting regime against postweaning oxidative stress, DNA damage, and hormonal disturbances for reducing mortality and morbidity in recently weaned male White New Zealand rabbits.
Growth Performance Traits
As shown in Table 3, there were no significant effects of fasting regimen and probiotics on final body weight and total body weight gain of growing rabbits. The study results agreed with those of El-Speiy et al. [29] and Beshara et al. [30], who repoted that probiotics did not influence rabbits’ daily weight gain in comparison with control animals. Mancini and Paci [16] reported that feeding restriction in growing rabbits (5–12 weeks) had no marked effect on body weight or daily weight gain. However, fasting of growing rabbits, with or without the addition of probiotics for 8 weeks postweaning, caused highly significant decreases in feeding intake by 38.5% and 33%, respectively, compared with the control group. The present results agreed with those of Sherif et al. [31], who found an extremely significant improvement in feed intake in the growing rabbits group with the ad libitum ones (control group) as compared with other feed restriction groups through all study intervals (5–13 weeks of age), and the FCR was highly significantly increased in growing rabbits with feed restriction system (FRS) (90% or 80%) in comparison with ad libitum animals during all periods of the experiment. No deaths occurred during the experimental period; these results agree with several previous studies which showed that restricted feeding did not affect the mortality rate in weaning rabbits [30, 32–34]. Other studies reported that long periods of restricted feeding (2 to 3 weeks) in growing rabbits decreased mortality and morbidity from digestive problems [2, 24]. Furthermore, previous studies documented that the viability of rabbits was improved from 2 to 16% when probiotics were added to their diet [21, 30].
Biomarkers of Serum Oxidative Status
The effects of fasting, probiotic supplementation, and fasting with probiotic supplementation on serum MDA, SOD, CAT, and TAC are shown in Table 4. Notably, growing rabbits during the fasting period had significantly elevated concentrations of serum MDA, an indicator of lipid peroxidation, by about 31% compared with rabbits in the control group; however, the lowest values were recorded in the ad libitum fed group in the presence of probiotic supplementation. This result may be due to severe nutrient deprivation, which causes increased oxidative stress as a result of the excessive production of ROS in mitochondria during cellular respiration; liver membranes are more sensitive to oxidative damage [35]. Nurmasitoh et al. [34], Kleniewska et al. [35], as well as Ayyanna and Ankaiah [36] reported that the lower percentage of fat in growing rabbits during fasting is probably one of the factors causing the increase in plasma MDA levels. However, serum SOD levels were significantly higher in the probiotic supplementation group (1143.6.0 mM/L) compared with other fasted groups with or without the addition of probiotics (1039.0 and 598.42 mM/L, respectively). The probiotic group had a slightly increased catalase level (CAT) and plasma TAC compared with other groups. These results may be due to reduced output of toxins or antimicrobial substances by other microorganisms, competition for adhesion to epithelial cells, increased resistance to colonization, prompting of the host’s immune system, and reduced stress in rabbits [16, 37].
Determination of Activity of Glutathione Peroxidase, Glutathione-S-Transferase, and Alkaline Phosphatase
Table 5 shows the activity levels of the antioxidant enzymes glutathione peroxidase (GPx) and glutathione-S-transferase (GST) in normal and fasted rabbits fed probiotics. The activity levels of GPx and GST in blood samples of fasted rabbits were reduced (5.3 ± 0.04 U/mg tissues/min and 2.1 ± 0.02 µmol/mg protein, respectively) compared with those in control rabbits (6.7 ± 0.07 U/mg tissues/min and 2.7 ± 0.03 µmol/mg protein, respectively). However, the GPx and GST activities were significantly (P < 0.05) increased (8.4 ± 0.11 U/mg tissues/min and 3.5 ± 0.06 µmol/mg protein, respectively) in rabbits supplemented with probiotics compared with fasted rabbits (5.3 ± 0.04 U/mg tissues/min and 2.1 ± 0.02 µmol/mg protein, respectively). Moreover, the activity levels of GPx and GST were increased (7.3 ± 0.03 U/mg tissues/min and 2.6 ± 0.04 µmol/mg protein, respectively) but without significant differences in fasted rabbits supplemented with probiotics compared with fasted rabbits (5.3 ± 0.04 U/mg tissues/min and 2.1 ± 0.02 µmol/mg protein, respectively). The probiotics have potential antioxidant effects [38, 39]. The ALP activities have no significant difference in all study groups in comparing with its level in control group.
Thyroid Hormones, Cortisol, and Glucose Levels
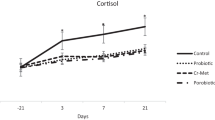
Triiodothyronine (T3) and thyroxin (T4) levels are shown in Table 6. The results display that there were no significant effects of the fasting regime and probiotics on serum T3 concentrations. However, plasma T4 concentrations were increased in the probiotic group with or without a fasting period when compared with those in the control and fasting groups [38]. This result may be due to feeding restrictions having a role in the regulatory mechanisms of metabolism in animals. Regarding plasma glucose and cortisol hormones (Table 6), the results did not show any significant differences (P < 0.01) between any experimental groups. However, there was a very small, nonsignificant decrease in cortisol levels in the fasted groups; this result was in agreement with Van Harten and Cardoso [39], who observed that blood glucose was not affected by restricted feeding in NZW rabbits. On the other hand, Rajman et al. [40] found a decrease in plasma glucose with a short (24 h) period of food restriction in chickens. Similarly, Jamshed et al. [41] and Grigorova et al. [42] observed that a slight restriction (50 g feed/head/day) reduced blood glucose during the feed restriction period.
Determination of DNA Damage
Table 7 shows the effect of probiotics feeding against starvation stress-induced DNA damage in male rabbits. The rate of DNA damage in fasted rabbits was increased (9.82 ± 0.86) significantly (P < 0.05) compared with control rabbits (6.80 ± 0.58). In contrast, supplementation of control or fasted rabbits with probiotics significantly decreased (P < 0.05) the rates of DNA damage (6.23 ± 0.80 or 7.41 ± 0.93, respectively) compared with those in fasted rabbits (9.82 ± 0.86). Ebeid et al. [43] and Uhlirova et al. [44] explained the positive protective effect of probiotics on DNA due to prevention of oxidative DNA damage and cellular oxidation as well as an antigenotoxicity effect.
Expression of ROS-Related Genes
Analysis of the expression of genes encoding antioxidant enzymes (CAT and SOD1) and ROS-producing genes (MDH1 and FMO2) in liver samples is illustrated in Figs. 1 and 2.
Alteration of the expression of catalase (CAT) and superoxide dismutase 1 (SOD1) genes in liver tissue samples of control and fasted rabbits fed a standard diet with probiotics. Means with different superscripts (a, b, c) between groups in the same treatment week are significantly different at P < 0.05. Data are presented as mean ± SEM
Alteration of the expression of MDH1 and FMO2 genes in liver tissue samples of control and fasted rabbits fed a standard diet with probiotics. Means with different superscripts (a, b, c) between groups in the same treatment week are significantly different at P < 0.05. Data are presented as mean ± SEM
The expression levels of CAT and SOD1 genes were significantly (P < 0.05) decreased in fatsted rabbits compared with those in control rabbits (Fig. 1). Conversely, the expression levels of CAT and SOD1 genes raised significantly (P < 0.05) in both fasted groups, and fasted rabbits fed a standard diet with the probiotic group compared with those in fasted rabbits.
On the other hand, the expression levels of MDH1 and FMO2 genes were significantly (P < 0.05) up-regulated in fasted rabbits compared with those in control rabbits (Fig. 2). In contrast, the expression levels of MDH1 and FMO2 genes decreased significantly (P < 0.05) in both of fasted group and fasted rabbits fed a standard diet with probiotic group compared with those in fasted rabbits. These results are in agreement with previous studies that documented that probiotics possess antioxidant and nephroprotective effects through elevation of the expression and activity of antioxidant enzymes [45,46,47,48].
Assessment of Apoptosis
Figure 3 shows the effect of probiotics against oxidative stress-induced apoptosis in fasted rabbits. The rates of necrosis and apoptosis in liver cells of fasted rabbits were increased considerably by 175.9% (15.3 ± 0.61) compared with those in control rabbits (8.7 ± 0.34). Nevertheless, the rates of necrosis and apoptosis in liver cells of rabbits supplemented with probiotics were close (9.2 ± 0.42) to those in control rabbits (8.7 ± 0.34). Additionally, fasted rabbits supplemented with probiotics exhibited a significant (P < 0.05) decline in the rates of necrosis and apoptosis (11.8 ± 0.67) in comparison with those in fasted rabbits (15.3 ± 0.61). Tiptiri-Kourpeti et al. [49] documented that probiotics exert antiproliferative effects. In addition, Blythe et al. [50] reported that intermittent fasting and consumption of probiotic yogurt are associated with immune modulation, detoxification, and antiproliferative effects.
Conclusion
This study showed that supplementation with probiotics accompanied by a fasting program significantly improved the antioxidant status of postweaning male White New Zealand rabbits by elevating the antioxidant indexes, such as MDA level and CAT, SOD, GSH-Px, and GST activities. Further, this improvement in serum antioxidant indexes seemed to be more effective than in the probiotics supplementation group. In addition, probiotics accompanied by a fasting program decreased the expression of ROS-related genes. Moreover, they could improve hormonal status in postweaning rabbits by reducing T3 and cortisol levels and increasing the level of glucose. This study suggests that supplementation with probiotics accompanied by a fasting program could be used in the future to alleviate oxidative stress, ROS, mortality, and morbidity, thereby contributing to the protective postweaning of rabbits.
Data Availability
All data and materials are available.
References
Gallois, M., Fortun-Lamothe, L., Michelan, A., & Gidenne, T. (2008). Adaptability of the digestive function according to age at weaning in the rabbit: II. Effect nutrient digestion in the small intestine and in the whole digestive tract. Animal, 2, 536–547. https://doi.org/10.1017/S1751731108001730
Gidenne, T., & Feugier, A. (2009). Feed restriction strategy in the growing rabbit 1 Impact on digestion, rate of passage and microbial activity. Animal, 3(4), 501–508. https://doi.org/10.1017/S1751731108003789
Tůmová, E., Volek, Z., Chodová, D., Härtlová, H., Makovický, P., Svobodová, J., Ebeid, T. A., & Uhlířová, L. (2016). The effect of 1-week feed restriction on performance, digestibility of nutrients and digestive system development in the growing rabbit. Animal, 10(1), 1–9. https://doi.org/10.1017/S1751731115001810
Xiccato, G., Trocino, A. (2010). Energy and protein metabolism and requirements, In The nutrition of the rabbit, 2nd edition (ed. C De Blas and J Wiseman), 83–118. CABI Publishing, Wallingford, UK. ISBN-13: 978 1 84593 669 3.
Al-Kawaz, J. M., & Jawad, N. M. (2022). Morphological and histological study of large intestine in adult local rabbits subjected to starvation. International Journal of Health Sciences, 6(S3), 2387–2402. https://doi.org/10.53730/ijhs.v6nS3.6051
Hermans, E. J., Henckens, M. J. A. G., Joëls, M., & Fernández, G. (2017). Time-dependent shifts in neural systems supporting decision-making under stress. Decision Neuroscience, 371–385. https://doi.org/10.1016/B978-0-12-805308-9.00030-0
Sirotkin, A. V., Koničková, I., Strup, O., Rafay, J., Laurincik, J., & Harrath, A. H. (2017). Caloric restriction and IGF-I administration promote rabbit fecundity: Possible interrelationships and mechanisms of action. Theriogenology, 90(2017), 252–259. https://doi.org/10.1016/j.theriogenology.2016.12.017
Muller, C. R., Leite, A. P. O., Yokota, R., Pereira, R. O., Americo, A. L. V., Nascimento, N. R. F., Evangelista, F. S., Farah, V., & Fonteles, M. C. P. (2019). Post-weaning exposure to high-fat diet induces kidney lipid accumulation and function impairment in adult rats. Frontiers in Nutrition. https://doi.org/10.3389/fnut.2019.00060
Novais, A. K., Deschêne, K., Martel-Kennes, Y., Roy, C., Laforest, J. P., Lessard, M., Matte, J. J., & Lapointe, J. (2021). Weaning differentially affects mitochondrial function, oxidative stress, inflammation and apoptosis in normal and low birth weight piglets. PLoS One, 16(2), e0247188. https://doi.org/10.1371/journal.pone.0247188
Chen, X. L., Gong, L. Z., & Xu, J. X. (2013). Antioxidative activity and protective effect of probiotics against high-fat diet-induced sperm damage in rats. Animal, 7(2), 287–292. https://doi.org/10.1017/S1751731112001528
Zheng, H. J., Guo, J., Jia, Q., Huang, Y. S., Huang, W. J., Zhang, W., Zhang, F., Liu, W. J., & Wang, Y. (2019). The effect of probiotic and synbiotic supplementation on biomarkers of inflammation and oxidative stress in diabetic patients: a systematic review and meta-analysis of randomized controlled trials. Pharmacological Research, 142, 303–313. https://doi.org/10.1016/j.phrs.2019.02.016.
Valavanidis, A., Vlachogianni, T., & Fiotakis, C. (2009). 8-Hydroxy-2′-deoxyguanosine(8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. Journal Environment Science Health C Environment Carcinogenesis Ecotoxicology Reviews, 27, 120–139. https://doi.org/10.1080/10590500902885684
Chung, K. F., & Marwick, J. A. (2010). Molecular mechanisms of oxidative stress in airways and lungs with reference to asthma and chronic obstructive pulmonary disease. Annals of the New York Academy of Sciences, 1203, 85–91. https://doi.org/10.1111/j.1749-6632.2010.05600.x
Caramori, G., Adcock, I. M., Casolari, P., Ito, K., Jazrawi, E., Tsaprouni, L., Villetti, G., Civelli, M., Carnini, C., Chung, K. F., Barnes, P. J., & Papi, A. (2011). Unbalanced oxidant-induced DNA damage and repair in COPD: A link towards lung cancer. Thorax, 66, 521–527. https://doi.org/10.1136/thx.2010.156448
Hong, H. A., le Duc, H., & Cutting, S. M. (2005). The use of bacterial spore formers as probiotics. FEMS Microbiology Reviews, 29(4), 813–835. https://doi.org/10.1016/j.femsre.2004.12.001
Mancini, S., & Paci, G. (2021). Probiotics in Rabbit Farming: Growth Performance, Health Status, and Meat Quality. Animals (Basel), 11(12), 3388. https://doi.org/10.3390/ani11123388
NRC. (2011). Guide for the care and use of laboratory animals. In National Academic Research Council Acad. http://www.nap.edu
Koracevic, D., Koracevic, G., Djordjevic, V., Andrejevic, S., & Cosic, V. (2001). Method for the measurement of antioxidant activity in human fluids. Journal Clinical Pathology, 4(5), 356–361. https://doi.org/10.1136/jcp.54.5.356
Richard, M. J., Guiraud, P., Monjo, A. M., & Favier, A. (1992). Development of a simple antioxidant screening assay using human skin fibroblasts. Free Radical Research Communications, 16(5), 303–314. https://doi.org/10.3109/10715769209049183
Miranda, M. V., Fernandez Lahor, H. M., & Cascone, O. (1995). Horseradish peroxidase extraction and purification by aqueous two-phase partition. Applied Biochemistry and Biotechnology, 53, 147–154. https://doi.org/10.1007/BF02788604
Abdel-Fattah, A. A., Sallam, K. M., & Moustafa, K. A. (2011). A novel method for determination of T3 and T4 hormones, associated with its physicochemical studies. Radiochemistry, 53, 213–220. https://doi.org/10.1134/S1066362211020172
Palme, R., Fischer, P., Schildorfer, H., & Ismail, M. N. (1996). Excretion of infused 14C steroid hormones via faeces and urine in domestic livestock. Animals Reproductive Science, 43, 43–63. https://doi.org/10.1016/0378-4320(95)01458-6
Blasiak, J., Arabski, M., Krupa, R., Wozniak, K., Zadrozny, M., Kasznikcki, J., Zurawska, M., & Drzewoski, J. (2004). DNA damage and repair in type 2 diabetes mellitus. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 554(1–2), 297–304. https://doi.org/10.1016/j.mrfmmm.2004.05.011
Cadet, J., & Davies, K. J. A. (2017). Oxidative DNA damage & repair: An introduction. Free Radical Biology & Medicine, 107, 2–12. https://doi.org/10.1016/j.freeradbiomed.2017.03.030
Olive, P. L., Banáth, J. P., & Durand, R. E. (2012). Heterogeneity in radiation-induced DNA damage and repair in tumor and normal cells measured using the “comet” assay. Radiation Research, 178(2), 35–42. https://doi.org/10.1667/rrav04.1
Hassan, S. K., El-Sammad, N. M., Abdel-Halim, A. H., Mousa, A. M., Khalil, W. K. B., & Anwar, N. (2018). Flavonoids-rich extract of beta vulgaris Subsp. cicla L. var. Flavescens leaf, a promising protector against gentamicininduced nephrotoxicity and hepatotoxicity in rats. International Journal of Pharmacology, 14(5), 652–666. https://doi.org/10.3923/ijp.2018.652.666
Alamoudi, E. F., Khalil, W. K. B., Ghaly, I. S., Hassan, N. H., & Ahmed, E. S. (2014). Nanoparticles from of Costus speciosus extract improves the antidiabetic and antilipidemic effects against STZ-induced diabetes mellitus in albino rats, International Journal of Pharmaceutical Sciences and Research, 29(1), 279–288. https://globalresearchonline.net/journalcontents/v29-1/52.pdf
Khalil, W. K. B., Zarouk, W., NourEldeen, G., Ramadan, A., Fayez, A., Esmaiel, N., Foda, B., Hamed, K., Kassem, S. M., & El-Bassyouni, H. (2019). Apoptosis, reactive oxygen species and DNA damage in familial Mediterranean fever patients. Gene Reports., 14(2019), 76–80. https://doi.org/10.1016/j.genrep.2018.11.010
El-Speiy, M. E., Kamel, K. I., El-Din, A. E. T., Abd-Hamid, A. E., & EL-Kamhawey, A. (2015). Effect of feed restriction on productive performance, carcass yield, blood pictures and relative organ weights of growing rabbits. Egyptian Poultry Science, 35, 439–454. https://doi.org/10.21608/epsj.2015.5372
Beshara, M. M., Alazab, A. M., Fahim, H. N., El Desoky, AEl. M. I., Ragab, M. A., El-Fhhat, A. M., & El-gamal, A. A. (2018). Effect of early dietary supplementation of probiotic and feed restriction post weaning on productive and economical performance of growing rabbits. Egyptian Journal of Rabbit Science, 28(1), 195–222. https://doi.org/10.21608/ejrs.2018.46510
Sherif, S. K., Elgohary, F. A., & Abo El-Maaty, H. A. (2019). Response to β-pro dietary supplementation in growing rabbits reared at different stocking densities under hot environmental conditions. Egyptian Poultry Science Journal, 39(I), 133–151. https://epsj.journals.ekb.eg/article_29134.html
Bhatt, R. S., Agrawal, A. R., & Sahoo, A. (2017). Effect of probiotic supplementation on growth performance, nutrient utilization and carcass characteristics of growing Chinchilla rabbits. Journal of Applied Animal Research, 45(1), 304–309. https://doi.org/10.1080/09712119.2016.1174126
Pizzino, G., Irrera, N., Cucinotta, M., Pallio, G., Mannino, F., Arcoraci, V., Squadrito, F., Altavilla, D., & Bitto, A. (2017). Oxidative Stress: Harms and Benefits for Human Health. Oxidative Medicine and Cellular Longevity, 2017, 8416763. https://doi.org/10.1155/2017/8416763
Nurmasitoh, T., Utami, S. Y., Kusumawardani, E., Najmuddin, A. A., & Fidianingsih, I. (2018). Intermittent fasting decreases oxidative stress parameters in Wistar rats (Rattus norvegicus). Univercity of Medicine, 37, 31–38. https://doi.org/10.18051/UnivMed.2018
Kleniewska, P., Hoffmann, A., Pniewska, E., & Pawliczak, R. (2016). The influence of probiotic lactobacillus casei in combination with prebiotic inulin on the antioxidant capacity of human plasma. Oxidative Medicine and Cellular Longevity, 2016, 1340903. https://doi.org/10.1155/2016/1340903
Ayyanna, R., & Ankaiah, D. V. (2018). Anti-inflammatory and antioxidant properties of probiotic bacterium lactobacillus mucosae an1 and lactobacillus fermentum SNR1 in wistar albino rats. Frontiers in Microbiology. https://doi.org/10.3389/fmicb.2018.03063
Kim, S., Lee, J. Y., Jeong, Y., & Kang, C. H. (2022). Antioxidant activity and probiotic properties of lactic acid bacteria, Fermentation, 8(1), 29. https://doi.org/10.3390/fermentation8010029
Spaggiari, G., Brigante, G., De Vincentis, S., Cattini, U., Roli, L., De Santis, M. C., Baraldi, E., Tagliavini, S., Varani, M., Trenti, T., Rochira, V., Simoni, M., & Santi, D. (2017). Probiotics ingestion does not directly affect thyroid hormonal parameters in hypothyroid patients on levothyroxine treatment. Frontiers in Endocrinology (Lausanne), 8, 316. https://doi.org/10.3389/fendo.2017.00316
Van Harten, S., & Cardoso, L. A. (2010). Feed restriction and genetic selection on the expression and activity of metabolism regulatory enzymes in rabbits. Animal, 4(11), 1873–1883. https://doi.org/10.1017/S1751731110001047
Rajman, M., Juráni, M., Lamosová, D., Mácajová, M., Sedlacková, M., Kost’ál, L., Jezová, D., & Výboh, P. (2006). The effects of feed restriction on plasma biochemistry in growing meat type chickens (Gallus gallus). Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 145(3), 363–371. https://doi.org/10.1016/j.cbpa.2006.07.004
Jamshed, H., Beyl, R. A., Della Manna, D. L., Yang, E. S., Ravussin, E., & Peterson, C. M. (2019). Early Time-Restricted Feeding Improves 24-Hour Glucose Levels and Affects Markers of the Circadian Clock. Aging, and Autophagy in Humans, Nutrients, 11(6), 1234. https://doi.org/10.3390/nu11061234
Grigorova, N., Ivanova, Z., Bjørndal, B., Berge, R. K., Vachkova, E., Milanova, A., Penchev, G., & Georgiev, I. P. (2022). Diet restriction alone improves glucose tolerance and insulin sensitivity than its coadministration with krill or fish oil in a rabbit model of castration-induced obesity. J Anim Physiol Anim Nutr (Berl)., 106(6), 1396–1407. https://doi.org/10.1111/jpn.13742
Ebeid, T., Tůmová, E., & Volek, Z. (2012). Effects of one week intensive feed restriction in the growing rabbit: Part-1 performance and blood biochemical parameters, World Rabbit Science Association. Proceedings 10 th World Rabbit Congress, 607–611. http://www.world-rabbit-science.com/WRSA-Proceedings/Congress-2012-Egypt/Papers/03-Nutrition/N-Ebeid.pdf
Uhlířová, L., Volek, Z., Marounek, M., & Tůmová, E. (2015). Effect of feed restriction and different crude protein sources on the performance, health status and carcass traits of growing rabbits. World Rabbit Science, 23(4), 263. https://doi.org/10.4995/wrs.2015.3229
de Oliveira Coelho, B., Fiorda-Mello, F., de Melo Pereira, G. V., Thomaz-Soccol, V., Rakshit, S. K., de Carvalho, J. C., & Soccol, C. R. (2019). In vitro probiotic properties and DNA protection activity of yeast and lactic acid bacteria isolated from a honey-based kefir beverage. Foods, 8(10), 485. https://doi.org/10.3390/foods8100485
Garcia-Gonzalez, N., Prete, R., Perugini, M., Merola, C., Battista, N., & Corsetti, A. (2020). Probiotic antigenotoxic activity as a DNA bioprotective tool: a minireview with focus on endocrine disruptors. FEMS Microbiology Letter, 367(3), fnaa041. https://doi.org/10.1093/femsle/fnaa041
Sengul, E., Gelen, S. U., Yıldırım, S., Çelebi, F., & Çınar, A. (2019). Probiotic bacteria attenuates cisplatin-induced nephrotoxicity through modulation of oxidative stress, inflammation and apoptosis in rats. Asian Pacific Journal of Tropical Biomedicine, 9(3), 116–122. https://doi.org/10.4103/2221-1691.254605
Sarwar, S., Hossain, M. J., Irfan, N. M., Ahsan, T., Arefin, M. S., Rahman, A., Alsubaie, A., Alharthi, B., Khandaker, M. U., Bradley, D. A., Emran, T. B., & Islam, S. N. (2022). Renoprotection of selected antioxidant-rich foods (water spinach and red grape) and probiotics in gentamicin-induced nephrotoxicity and oxidative stress in rats. Life (Basel), 12(1), 60. https://doi.org/10.3390/life12010060
Tiptiri-Kourpeti, A., Spyridopoulou, K., Santarmaki, V., Aindelis, G., Tompoulidou, E., Lamprianidou, E. E., Saxami, G., Ypsilantis, P., Lampri, E. S., Simopoulos, C., Kotsianidis, I., Galanis, A., Kourkoutas, Y., Dimitrellou, D., & Chlichlia, K. (2016). Lactobacillus casei exerts anti-proliferative effects accompanied by apoptotic cell death and up-regulation of TRAIL in colon carcinoma cells. PLoS. https://doi.org/10.1371/journal.pone.0147960
Blythe, J., Ruggiero, M., & Pacini, S. (2017). Intermittent fasting and probiotic yogurt consumption are associated with reduction of serum alpha-N-acetylgalactosaminidase and increased urinary excretion of lipophilic toxicants. Madridge Journal of Immunology. https://doi.org/10.18689/mjim-1000107
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors have contributed equally in this work.
Corresponding author
Ethics declarations
Ethical Approval
This study was approved by the Institutional Animal Care and Use committee of Cairo University, protocol No. (CU-II-F37-17).
Conflict of Interests
The authors declare that they have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abou-Hashim, F., Khalifa, W.H., Shalaby, M.B. et al. Evaluation of Fasting and Probiotics in Reducing Postweaning Stress in Rabbits: Study of their Effects on Biochemical and Gene expression Patterns. Appl Biochem Biotechnol 196, 558–572 (2024). https://doi.org/10.1007/s12010-023-04479-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04479-w