Abstract
COVID-19 infection is a new disease and our knowledge is limited; day in and day out more and more interesting yet diverse observations are reported by the different research groups from different corners of the world. So, there is an urgent requirement of the invention of some effective and efficient drugs that can carry out the end of the deadly viral infection. Throughout the world, there have been many efforts carried out in different labs to invent such a drug and also identifying any pre-existing drugs which can carry out the killing of the virus. In this review, an effort has been made to understand the potential drugs which can be used against the SARS-CoV-2 viral infection. Again, the strategies on the current and the future drug discovery mechanisms against the SARS-CoV-2 are also mentioned. The different drugs made and the drugs re-used and also the drugs which are in the making process in different research laboratories across the world are also mentioned. To combat this unexpected crisis, we still need some more efforts from the different scientific communities around the world for finding a cure against this viral infection and this is needed to be done for the prevention of more loss of human life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
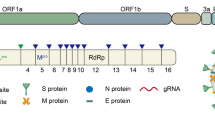
The virus SARS COV2 emerged and spread across the world like a conflagration in no time. Initially, countries of East Asia such as China, South Korea and Japan were affected, then it spread to other Asian countries. It reached the West Asian countries and Iran became a hotspot of the COVID-19 pandemic. It made its advancement towards the European countries. In the following months, it spread across the Atlantic and both North and South America were under the grip of this global pandemic. The USA, Brazil, Russia, India, and the countries of Europe are among the worst hit countries. Except a few island nations, almost all the countries and territories are now more or less affected by this pandemic. Few countries such as Italy, Spain, and Mexico are showing high death rate compared to other countries. Symptoms of this disease are much like infection due to influenza virus (Fig. 1). It is probably a zoonotic virus which has many probable sources (Fig. 2). The guidelines of using different drugs for COVID treatment have been revised several times since inception. These revisions and inclusion or exclusion of the drugs from the list are completely based on the outcomes of the clinical studies [1, 2]. The dose and time span of taking these drugs differ significantly from one to another. IFN-α is a broad-spectrum antiviral drug which is used to treat hepatitis. Recent in vitro studies have shown that it has the capacity to inhibit SARS-CoV-2. Lopinavir or ritonavir is a common drug for treatment of HIV infection [3]. Both in vitro and clinical studies have revealed that lopinavir/ritonavir has anti-SARS-CoV-2 activities [4]. Ribavirin is a nucleoside analogue and is used to treat different viral infections. A study based on clinical trial on patients with severe acute respiratory syndrome revealed that a combined dose of lopinavir/ritonavir and ribavirin was more effective in combating infection compared to that of ribavirin mono-therapy [5]. Chloroquine is widely used for the treatment of malaria and later it was also found to have antiviral effects [6]. It was reported that administration of very low concentration of it can inhibit or resist SARS CoV-2 infection [7]. Arbidol is also an antiviral drug that is used to inhibit the influenza virus. A recent in vitro study reports that arbidol can effectively resist SARS-CoV-2 infection at a concentration of 10–30 μM [8]. All these drugs are mentioned in the guidelines circulated by WHO. Favipiravir is another drug which has grabbed the attention of the scientists and medical practitioners as potential candidate for COVID treatment. Favipiravir was initially approved for treatment of COVID-19 in February this year in China [9]. As it is a type of RNA-dependent RNA polymerase inhibitor, it has the potential to inhibit COVID-19 which is a RNA virus. It is already established that besides its antiviral activity, it is also capable of blocking the replication of different RNA viruses because it has RNA-dependent RNA polymerase inhibition activity. Within the cells, Favipiravir is converted into an active phosphoribosylated form (Favipiravir-RTP) and helps in inhibiting RNA polymerase activity [10]. All these findings make it a potential candidate in thwarting SARS-CoV-2 infection. A recent clinical study has revealed that Favipiravir has more antiviral action than that of lopinavir/ritonavir [11]. This study was conducted on 80 patients; no adverse reactions were noted in patients who were treated with Favipiravir. In vivo studies on mice model have shown that Remdesivir can effectively act against MERS-CoV infection and also helps to improve the lung. Several clinical studies reported the positive impact of Remdesivir in treating SARS-CoV-2 infection [10]. Clinical trials are also conducted to test the efficacy and safety of this drug for treatment of COVID-19 [12]. Studies have revealed that some other drugs can also be effective in treating COVID-19. Darunavir is one of those; it is actually a second-generation HIV-1 protease inhibitor [13]. In vitro experiments in animal cell line indicated that Darunavir is capable of inhibiting viral replication [14]. SARS-CoV-2 enters the target cell by interacting with SARS-CoV-2 receptor and the cellular protease TMPRSS2 helps in this process. A TMPRSS2 inhibitor would block the entry of the virus and therefore can be a potential treatment option. Other drugs including type II transmembrane serine protease inhibitors and BCR-ABL kinase inhibitor imatinib can be considered as potential drugs for COVID treatment [12]. As development of vaccines against this COVID-19 is going to take time and even if it is produced and sanctioned for human use, the amount of vaccines needed to vaccinate the entire human race will be huge, hence it will be a time-consuming affair. In this circumstance, the only alternate is to keep on using these available drugs and try to formulate a standard treatment protocol to treat patients infected with this virus. There are about 60 antiviral drugs which are under clinical trials for treatment of COVID-19. The efficacy and safety of these candidate drugs are yet to be confirmed [14].
Use of Antiviral Drugs for COVID 19 Treatments
There are many antiviral drugs used to treat different viral infections. Research is going on to evaluate whether these drugs can have the potential to be used for COVID treatment [15]. There are several such molecules, for example the interferon alpha, beta and gamma, ribavirin, etc. Their pharmacokinetic and pharmacodynamic properties are well known and documented [16]. Although these drugs are not produced to treat specifically COVID-19 virus but they might have the potential to inhibit and thwart SARS-CoV-2 viral infection [17].
High-Throughput Screening of Anti-viral Compounds
The capability of different antiviral compounds to have anti-COVID-19 properties can be examined by in vitro cell line studies [18]. Highly efficient screening technologies are used to check the capability of different chemical compounds to inhibit SARS COV-2 infection. In silico approach with the help of bioinformatical data sets of the available drugs is now extensively studied to elucidate the possibility of repurposing the use of these drugs for COVID-19 treatment which in turn can lead to discovery of many new roles of some selected drug molecules [10]. There are already some drugs in the market like lopinavir/ritonavir which were actually produced for the treatment of HIV. The possibility of using these drugs for treating SARS-COV-2 infection was investigated. But surprisingly, the outcome of the research showed a totally different pattern which is contrary to the predictions. There can be several reasons behind this [10]. One of the major reasons may be that at higher concentration, these drugs show immunosuppressive and cytotoxic effects [19].
Approaches for Drug Repurposing
New drug discovery and elucidating new ways of drug delivery are a time-consuming process; under the current prevailing situation where time is the main constraint [19]. Although our aim should be to design a novel drug which should be specifically designed to act against COVID-19 virus, but under this present circumstance, we should also keep on analyzing the potentiality of different other drugs as a candidate drug to treat COVID-19 infection by the process of drug repurposing as this is a comparatively less time-consuming affair (Fig. 3). Hence, the medical practitioners and pharmaceutical companies are now focusing on drug re-purposing besides the discovery of new target specific drug [16]. Repeated clinical trials are conducted to determine its efficacy. Several other related terms such as drug repositioning, drug re-profiling or drug retasking are also used besides the common term drug repurposing [20]. During this process, three phases of clinical trials are conducted to not only examine its efficiency but also to monitor whether the drugs are having any toxic effect on the patients or the drugs are causing any other negative impact on patients. Hence, it reduces the chances of health hazards in patients and helps to formulate safety measures. These repurposed drugs can skip the phase I and II of clinical trials because they are already used for treating other diseases and based on the results of their performance on the phase III, they can be launched into the market [21].
In silico Approach
Bio-computational or bio-informatics processes play a huge role in drug repurposing. Screening of database related to gene expression, chemical structure, proteomic data or electronic healthcare history is used for this purpose. Among the many different approaches signature matching computational molecular docking, genomic association analysis is a few to name. Apart from that cellular mechanism study or mapped networks and even some retrospective detection approach which uses historical data of approved drugs are also considered [20]. Signature matching or unique character: Each and every drug has a unique character or structural novelty. When a drug is being made or developed, it should have a unique character or signature like its transcriptomic effect profile, structural or adverse effect profile and thereby matching or linking these characters, repurposing can be done. Now using this approach for drug repurposing, the researchers actually believe in drug or drug disease comparison [21]. Now on the very first time, the gene expression of a drug’s usage before and after is being compared with the differential gene expression obtained by comparing other profiles of healthy individuals with the diseased ones [22]. One example of drug repurposing is the topiramate which is an antiepileptic drug and has an antagonistic effect for GABA. Research has also shown that this topiramate can be used for inflammatory bowel disease because of its signature characteristics [23, 24]. Computational molecular docking: This is a very important and indispensable tool for drug repurposing activity. Here we use structure-based computational strategy, through which the binding efficiency is predicted between the drug and the target molecule [25]. However, each and every technique in biological sciences has some or the other disadvantages or limitations [26].
Network Mapping
Many drug targets that are identified may not be directly targeted because their direct inhibition can actually lead to some serious effects and this network or molecular pathway is done which will give us an idea about the upstream and downstream targets (Table 1) [36]. Drug and disease connection can be done or created using this network mapping and therefore can open huge possibilities for drug repurposing. Artificial intelligence and drug repurposing: With helping machine learning tools, computational algorithms can be developed which will thereby help in predicting newer drug target sites and thus with greater accuracy than the earlier methods [37]. Huge data that has been generated by high-throughput Next Gen Sequencing from a number of patients, when combined with the characteristics of the diseases and treatments can thereby lead to the identification of the new biomarkers that can be utilized. Artificial Intelligence–supervised machine learning algorithms can be used to implement multiomics and multitask learning to facilitate the drug response by engaging multiple drugs [38]. For example, recently a study has been done in which a methodology was developed that can use heterogeneous data derived from previously described drug target interaction to predict new interactions with even greater efficiency [39]. This method is known as ‘deep DTnet’ that can integrate multiple drugs with drug targets and diseases with the help of deep learning.
Pharmacological Interventions
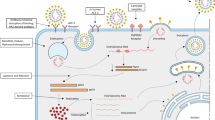
Lessons that have been obtained from SARS and MERS epidemic have helped us in developing some therapies against SARS-CoV-2. Drugs that are used previously for the treatment of viral infection like oseltamivir, peramivir, zanamivir, ganciclovir, acyclovir and ribavirin are not recommended for COVID-19 treatment. Also, systemic corticosteroid treatment such as methylprednisolone cannot be used as a treatment for SARS-CoV-2 infected patients. The similarity between the MARS, SARS and SARS-COV-2 provides more encouragement for drug discovery and repurposing [40]. As of now, there is no specific antiviral drug which can be used solely for Covid-associated pathologies. But some of the drug target sites have been identified which if targeted can control the disease [41]. Since the previous global corona virus pandemics like MERS and SARS have come into existence, hence considerable amount of research has been done for suitable drug targets and subsequent drug candidates. Inhibition of SARS-CoV-2 fusion and entry: SARS-COV-2 uses the viral spike protein to make its entry into the host cells and therefore causes several protein interactions which will thereby take place between the spike protein of SARS and in the region of ACE2 receptor and thereby can be targeted for drug action [40]. Like other viral infection, the coronavirus also causes mutation specifically in the spike protein and thereby it cannot be its coveted function. As a result of mutation, the spike protein recognizes the ACE2 receptor much more efficiently and thus the previously studied Receptor Bonding domain is the target for the drugs [42]. Another strategy that can be developed is to engage the ACE2 receptor with recombination ACE2 receptor which is normally present on the cell surface and thus excess ACE2 will thereby cause the neutralization of the virus by competitively binding to SARS-CoV-2 (rhACE2; GSK2586881) [43]. In addition to this, the HR1 and HR2 present on the SARS-CoV-2 have also been implicated in the facilitation of cell membrane fusion. HR2–derived peptides exhibit effective fusion inhibitory activity [32].
Inhibition of Endocytosis
After the fusion between the spike and ACE2 protein, the virus is ingested in the cells in a pH and receptor–dependent endocytosis [44]. Targeting the endocytosis can be one of the mechanisms of inhibiting the disease and thereby can be a target site for developing drugs. Clathrin-mediated endocytosis is regulated by AP-2-associated protein kinase 1. Based on a library screening process, JK inhibitor Baricitinib was identified as a possible drug candidate for SARS-COV-2 and also oubain can be used as an inhibitor of clathrin mediated, is also being tested for its effectiveness in drug trials for SARS-COV-2 positive patients. Recently, Chloroquine and its derivative hydroxychloroquine have gained popularity as a therapy against SARS-CoV-2 infection [45, 46]. Inhibition of viral enzymes: like Lopinavir and Ritonavir are protease inhibitors that target 3CLproof SARS-CoV-2. The main protease, 3CLpro, is responsible for processing the polypeptide to NSPs. By utilizing high-throughput screening for compounds against 3CLpro, four molecules, viz. Prulifloxacin, Tegobuvir, Bictegravirand and Nelfinavir, were identified [47].
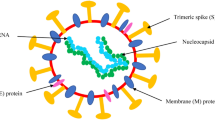
Inhibition of viral envelope, membrane, nucleocapsid and accessory proteins: SARS-CoV-2 envelope (E), membrane (M) and nucleocapsid (N) protein are extremely necessary for virus survival and propagation, and therefore such structural proteins are the best drug targets. Since these proteins are structurally different from the host proteins, hence, the drugs targeting these proteins will have minimal effect. These proteins help in protecting the viral genome and therefore these structural proteins are involved in suppressing the immune system and therefore the virus has an additional advantage over the host cell [48]. The N protein helps in suppressing the RNA interference and hence many siRNA-based treatments can target viral E, M, N and hence can inhibit protein translation in vivo [49]. Suppression of excessive inflammatory response: A well cytokine response is absolutely critical for the host immune response. It has been reported that patients suffering from Covid-19 disease show a hyper inflammatory reaction due to deregulation of cytokine receptor [50]. SARS-CoV-2 infected patients admitted in ICU display increased levels of GM-CSF and IL6 CD4T cells when compared to non-ICU patients [51]. Thereby, it can be said that inhibition in the inflammatory response can reduce the severity of COVID-19 disease. But their use in COVID-19 patients is still unsure and requires a detailed study but it has been demonstrated that after the COVID-19 infection, the CD4T cells are activated to produce GM-CSF and other inflammatory cytokines, thereby resulting in further induction of CD14 CD16 monocytes with high expression of IL-6 [52]. Companies actively involved in finding a drug to treat SARS-CoV-2 patients are listed in Table 2.
Drugs Which Are Developed and Used Against the SARS-COV-2 Viral Outbreak
Here are some of the most searched or experimented drugs about the treatment for the corona virus. But despite all these, most of the drugs are on their early stage of research. Now there are certain labels that have been given by the scientists [59]. These are the treatments that have been used widely by the doctors and nurses to treat the patients suffering from Covid-19 and also to the patients having cardiovascular diseases. Promising evidence: This label includes treatments that have responded improvements in morbidity, mortality and recovery in at least one random controlled trial, in which some people will get a treatment and others get a place [60]. Tentative or mixed evidence: This is the label where treatments showed promising results in cells or animals, but that are yet to be confirmed in people. Some treatments have produced different results in different experiments when performed, thereby raising confusion or a need for more rigorous studies to remove the confusion whether these treatments will be helpful in humans or not. Not promising: These are the treatments which give us an idea about the fact that these treatments do not work. Evidence in cell lines, animal models or human trials: These labels give us an idea about where the evidence for a treatment will come from. Researchers first start their experiments on cells and then move onto higher order of animals. Many of these animal experiments often fail but if they get promising results, researchers then move to humans, such as random clinical trials. Sometimes, scientists try out other drugs to treat patients with COVID-19 infection which were meant for the treatment of other diseases. Drug which gave the most promising evidence is Remdesivir, made by Gilead Sciences and was also the first drug to get emergency authorization from the FDA for use on Covid-19. Remdesivir has the capability of interfering or inserting them within the new viral genes and thereby helps in controlling the disease. This move was criticized by some of the experts who said that FDA had expanded remdesivir’s use without strong evidence to back the change [61]. Another drug that gave a promising result is MK-4482 which was made to fight flu, MK-4482 (previously known as EIDD-2801). This drug gave promising results against the corona virus when it was studied in cells and on animals. Merck has said that a large phase three trial will be launched in the month of September. Recombinant ACE2 proteins: - Corona virus has a very strong affinity towards ACE2 protein and thereby forms a recombinant. Some scientists believe that this recombinant formed will decrease the rate of the infection and thus will lure the virus away from vulnerable cells [62]. This experiment has shown promising results on cells but not in humans and animals.
Ivermectin
For more than 20 years, this potent drug has shown its effect against parasitic worms. Doctors have used it against river blindness, while veterinarians have used it against heartworm in dogs. But when it has been studied in cells, this drug has shown a possibility of killing virus also [62]. But this is not yet checked or tested by a researcher or scientist whether this drug will be a potent antiviral. It has been seen that this drug has the capacity to control Corona virus in cells but the amount of dosage given is so high that it will yield a dangerous effect on an individual’s health and thereby can lead to some serious harm. Hence, FDA has issued some guidelines or warnings against the usage of this drug [63, 64].
Hydroxychloroquine and Chloroquine
Chloroquine is the drug which was originally used against malaria and the lesser toxic version the hydroxychloroquine was used against lupus and arthritis. But during the initial stages of COVID-19 pandemic, these drugs have proved to be limiting or inhibiting the replication of virus in the cells and this was given to patients suffering from Covid-19. But when it has been studied in detail, it has been seen that studies that have been done on mice and monkey have no results and thus will not help in preventing the disease and thus the trials were halted as it can cause some serious harm to one’s health [65]. Despite getting some negative results, trials were done but in a very small scale and thus it can be given to patients theoretically in early stages of this disease and thus only well and proper designed trials can determine this [66]. Hydroxychloroquine therapy is not associated with liver function abnormalities. It is also known as oxychlorochin which belongs to the class of organic compound known as 4-aminiquinolines. These organic compounds are containing an amino group attached to the fourth position of a quinoline ring. Studies show the outcome molecular quantum mechanical modelling of the interaction of hydroxychloroquine to the SARS-COV-2 spike protein-ACE2 complex [67]. ACE-2 is the critical receptor of SARS-COV-2 virus; the principle is inhibiting this interaction with a suitable drug. According to the molecular modelling and study, hydroxychloroquine does not bind to ACE-2 but increases its acidity in the interaction between the ACE-2 and the spike protein; thus the degradation in the spike and lowering the ability to spread the virus [68]. Hydroxychloroquine is the first metabolite of chloroquine [69]. Besides this, it also helps in raising the pH within the endosomes and lysosomes and interfering with viral infection and major role of hydroxychloroquine is the inhibition three key pro-inflammatory cytokines tumor necrosis factor alpha, interieukin1, and interieukin6 produced from the immune response from mononuclear phagocytes in response of to the viral infection [70]. At present, almost all the countries have stopped using this drug for COVID treatment.
Dexamethasone and Other Corticosteroids
Corticosteroids are often called steroids in short that can be used to tamp down inflammation and can also be given to a person suffering from conditions such as allergies and asthma [71]. Thus, doctors began using these drugs as a treatment for pneumonia and other severe respiratory illnesses, but the clinical trial results were inconclusive [72, 73].
Cytokine Inhibitors
Human body produces some important molecules called cytokines to fight against diseases and one most important feature of this cytokine molecule is that they can cause cytokine storm and thereby researches have made some drugs which can halt these storms and these drugs have proved to be effective against arthritis and other inflammatory diseases [74]. Now against the corona virus, some drugs have given modest help during trials, two drugs called sarilumab and tocilizumab are in use which will both target the cytokine IL- [75].
Favipiravir
The National Medical Products Administration (NMPA) has approved the usage of Favipiravir which is an antiviral drug; it can be used for corona virus treatment [76, 77]. The drug has shown its efficacy in treating the disease with fewer side effects.
Few Other Potential Drugs Which Are Still Under Manufacturing Process
Gimsilumab by Roivant Sciences
It is a human monoclonal antibody which is in its clinical trial stage. The drug targets GM-CSF, which is an inflammatory cytokine found in high levels in the serum of COVID-19 patients [78].
AdCOVID by Altimmune
Anti-immune has made collaboration with the University of Alabama to develop an intranasal vaccine named AdCOVID for corona patients [79].
TJM2 by I-Mab Biopharma
I-Mab Biopharma is being developed by TJM2 which is a neutralizing antibody which will treat the cytokine storm that occurs in patients suffering from severe corona virus case [80]. This drug targets the GM-CSF, which is responsible for acute and chronic inflammation. The Infectious Disease Research Centre is going to develop antibodies against SARS-CoV-2.
AT-100 by Airway Therapeutics
Airway Therapeutics is making or developing a human recombinant protein named AT-100 (rhSP-D) as a treatment for corona virus [81]. This protein AT-100 has shown efficacy in preclinical trials in reducing the inflammation and infection in the lungs, while also performed by generating an immune response against various respiratory diseases.
TZLS-501 by Tiziana Life Sciences
Tiziana Life Sciences is developing a monoclonal antibody named TZLS-501 for the treatment of COVID-19. TZLS-501 is a human anti-interleukin-6 receptor, which helps in preventing lung damage and elevated levels of IL-6 [82].
OYA1 by OyaGen
OyaGen has developed a drug called OYA1 which has shown strong antiviral efficacy against coronavirus in vivo [83]. OYA1 was also earlier approved for treating cancer but was withdrawn due to lack in efficacy. OyaGen plans to conduct further research on this drug to determine the efficacy in treating the coronavirus.
BPI-002 by BeyondSpring
BeyondSpring’s BPI-002 is a small molecule agent which has been developed for treating various infections including COVID-19. It has the ability to activate CD4+ helper T cells and CD8+ cytotoxic T cells and generating an immune response in the body. Now if combined with another COVID-19 vaccine, the efficacy of the drug increases and thus was able to generate long-term protection against viral infections [84].
NP-120 (Ifenprodil) by Algernon Pharmaceuticals
Algernon Pharmaceuticals is now busy in exploring the drug Ifenprodil as a potential treatment for COVID-19 [85, 86]. Ifenprodil is an N-methyl-d-aspartate receptor glutamate receptor antagonist which was sold under the brand name Cerocal. It has showed efficacy in increasing the survivability in mice infected with H5N1 influenza.
APN01 by APEIRON Biologics
A drug was developed by APEIRON Biologics named APN01 which is being tested in China in a phase one trial as a treatment against COVID-19 [87, 88]. The clinical trial in China will thereby give an idea about the efficacy of the drug and agree in obtaining the data; it will be decided whether the experiment should be conducted in patients.
Brilacidin by Innovation Pharmaceuticals
Innovation Pharmaceuticals is evaluating Brilacidin which is a defense mimetic drug which has a potential in treating the coronavirus. Brilacidin has surprisingly shown many properties in several trials like antibacterial, anti-inflammatory and immune modulatory properties and now the company is aiming for exploring research collaboration and also to seek grant for manufacturing the drug [89]. A brief list of potential drugs for short-term treatment of COVID 19 infection is given in Table 3.
Conclusion
Even after 3 years of rigorous research, we do not even have enough information about the structure of the virus, its nature, mutations, etc. Several studies show that severity of symptoms in COVID-19 can be categorised into mild, moderate, and severe. In moderate and severe cases, availability of adequate oxygen support, appropriate and timely administration of anti-coagulants and widely available and inexpensive corticosteroids in accordance with the protocol can be considered as the backbone of COVID-19 therapy. Among these drugs, the broad spectrum antiviral medications is considered more effective. Till the time we have a particular targeted drug against COVID-19, we have to rely on the available drugs and use different combinations of those drugs to treat the affected individuals depending on the severity and manifestation of infection.
References
Alexander, R., Ravi, A., Barclay, H., Sawhney, I., Chester, V., Malcolm, V., Brolly, K., Mukherjee, K., Zia, A., Tharian, R., Howell, A., Lane, T., Cooper, V., & Langdon, P. E. (2020). Guidance for the treatment and management of COVID-19 among people with intellectual disabilities. Journal of Policy and Practice in Intellectual Disabilities, 17(3), 256–269. https://doi.org/10.1111/jppi.12352
Dong, Y., Mo, X., Hu, Y., Qi, X., Jiang, F., Jiang, Z., & Tong, S. (2020). Epidemiology of COVID-19 among children in China. Pediatrics, 145(6), e20200702.
Zhou, P., Yang, X. L., Wang, X. G., Hu, B., Zhang, L., Zhang, W., Si, H. R., Zhu, Y., Li, B., Huang, C. L., Chen, H. D., Chen, J., Luo, Y., Guo, H., Jiang, R. D., Liu, M. Q., Chen, Y., Shen, X. R., Wang, X., et al. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature, 579(7798), 270–273.
Chu, D. K., Akl, E. A., Duda, S., Solo, K., Yaacoub, S., & Schünemann, H. J. (2020). COVID-19 Systematic Urgent Review Group Effort (SURGE) study authors. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet, 395(10242), 1973–1987.
Harcourt, J., Tamin, A., Lu, X., Kamili, S., Sakthivel, S. K., Murray, J., Queen, K., Tao, Y., Paden, C. R., Zhang, J., Li, Y., Uehara, A., Wang, H., Goldsmith, C., Bullock, H. A., Wang, L., Whitaker, B., Lynch, B., Gautam, R., et al. (2020). Severe acute respiratory syndrome coronavirus 2 from patient with coronavirus disease United States. Emerging Infectious Diseases, 26(6), 1266–1273.
Ahn, D. G., Shin, H. J., Kim, M. H., Lee, S., Kim, H. S., Myoung, J., Kim, B. T., & Kim, S. J. (2020). Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID-19). Journal of Microbiology and Biotechnology, 30(3), 313–324.
Hijikata, A., Shionyu-Mitsuyama, C., Nakae, S., Shionyu, M., & Ota.M, Kanaya. S, Shirai T. (2020). Knowledge-based structural models of SARS-CoV-2 proteins and their complexes with potential drugs. FEBS Letters, 594(12), 1960–1973.
Mara, A., Jones, S. J. M., Astell, C. R., & Holt, R. A. (2003). The Genome sequence of the SARS-associated coronavirus. Science, 300(5624), 1399–1404.
Jeon, S., Ko, M., Lee, J., Choi, I., Byun, S. Y., Park, S., Shum, D., & Kim, S. (2020). Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs. Antimicrobial Agents and Chemotherapy, 64(7), e00819–e00820.
Omari, O., Sabei, S., Rawajfah, O., Abu Sharour, L., Aljohani, K., Alomari, K., Shkman, L., Dameery, K., Saifan, A., Zubidi, B., Anwar, S., & Alhalaiqa, F. (2020). Prevalence and predictors of depression, anxiety, and stress among youth at the time of COVID-19: An online cross-sectional multicountry study. Depression Research and Treatment, 6(2020), 8887727.
Hsih, W. H., Cheng, M. Y., Ho, M. W., Chou, C. H., Lin, P. C., Chi, C. Y., Liao, W. C., Chen, C. Y., Leong, L. Y., Tien, N., Lai, H. C., Lai, Y. C., & Lu, M. C. (2020). Featuring COVID-19 cases via screening symptomatic patients with epidemiologic link during flu season in a medical center of central Taiwan. Journal of Microbiology, Immunology and Infection, 53(3), 459–466.
Kandeel, M., Ibrahim, A., Fayez, M., & Al-Nazawi, M. (2020). From SARS and MERS CoVs to SARS-CoV-2: Moving toward more biased codon usage in viral structural and nonstructural genes. Journal of Medical Virology, 92(6), 660–666.
Hoffmann, M., Kleine-Weber, H., Schroeder, S., Krüger, N., Herrler, T., Erichsen, S., Schiergens, T. S., Herrler, G., Wu, N. H., Nitsche, A., Müller, M. A., Drosten, C., & Pöhlmann, S. (2020). SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell, 181(2), 271–280.
Brown E, Gray R, Lo Monaco S, O’Donoghue B, Nelson B, Thompson A, Francey S, McGorry P. The potential impact of COVID-19 on psychosis: A rapid review of contemporary epidemic and pandemic research. Schizophr Res. 2020 Aug;222:79-87. Doi: 10.1016/j.schres.2020.05.005. Epub 2020 May 6. PMID: 32389615; PMCID: PMC7200363.
Agarwal, A., Mukherjee, A., Kumar, G., Chatterjee, P., Bhatnagar, T., & Malhotra, P. (2020). PLACID trial collaborators. Convalescent plasma in the management of moderate Covid-19 in adults in India: Open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ, 371, m3939.
Van Boheemen, S., de Graaf, M., Lauber, C., Bestebroer, T. M., Raj, V. S., Zaki, A. M., Osterhaus, A. D., Haagmans, B. L., Gorbalenya, A. E., Snijder, E. J., & Fouchier, R. A. (2012). Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. mBio, 3(6), e00473–e00412.
Benvenuto, D., Giovanetti, M., Ciccozzi, A., Spoto, S., Angeletti, S., & Ciccozzi, M. The 2019-new coronavirus epidemic: Evidence for virus evolution. Journal of Medical Virology, 92(4), 455–459.
Xia, S., Liu, M., Wang, C., Xu, W., Lan, Q., Feng, S., Qi, F., Bao, L., Du, L., Liu, S., Qin, C., Sun, F., Shi, Z., Zhu, Y., Jiang, S., & Lu, L. (2020). Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Research, 30(4), 343–355.
Yin, I. X., Zhang, J., Zhao, I. S., Mei, M. L., Li, Q., & Chu, C. H. The antibacterial mechanism of silver nanoparticles and its application in dentistry. International Journal of Nanomedicine, 15, 2555–2562.
Rehman, U., Shahnawaz, M. G., Khan, N. H., Kharshiing, K. D., Khursheed, M., Gupta, K., Kashyap, D., & Uniyal, R. (2021). Depression, anxiety and stress among Indians in times of Covid-19 lockdown. Community Mental Health Journal, 57(1), 42–48.
Alanagreh, L., Alzoughool, F., & Atoum, M. (2020). The human coronavirus disease COVID-19: Its origin, characteristics, and insights into potential drugs and its mechanisms. Pathogens, 9(5), 331.
Coutard, B., Valle, C., de Lamballerie, X., Canard, B., Seidah, N. G., & Decroly, E. (2020). The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Research, 76, 104742.
Rajkumar, R. P. (2020). COVID-19 and mental health: A review of the existing literature. Asian Journal of Psychiatry, 52, 102066.
Li, Q., Guan, X., Wu, P., Wang, X., et al. (2020). Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. New England Journal of Medicine, 382, 1199–1207.
Glebov, O. O. (2020). Understanding SARS-CoV-2 endocytosis for COVID-19 drug repurposing. The FEBS Journal, 287(17), 3664–3671.
Nieto-Torres, J. L., Dediego, M. L., Alvarez, E., Jiménez-Guardeño, J. M., Regla-Nava, J. A., Llorente, M., Kremer, L., Shuo, S., & Enjuanes, L. (2011). Subcellular location and topology of severe acute respiratory syndrome coronavirus envelope protein. Virology, 415(2), 69–82.
Xia, S., Liu, Q., Wang, Q., Sun, Z., Su, S., Du, L., Ying, T., Lu, L., & Jiang, S. (2014). Middle East respiratory syndrome coronavirus (MERS-CoV) entry inhibitors targeting spike protein. Virus Research, 194, 200–210.
Mukherjee, M., Forero, D. F., Tran, S., Boulay, M. E., Bertrand, M., Bhalla, A., Cherukat, J., Al-Hayyan, H., Ayoub, A., Revill, S. D., Javkar, T., Radford, K., Kjarsgaard, M., Huang, C., Dvorkin-Gheva, A., Ask, K., Olivenstein, R., Dendukuri, N., Lemiere, C., et al. (2020). Suboptimal treatment response to anti-IL-5 monoclonal antibodies in severe eosinophilic asthmatics with airway autoimmune phenomena. European Respiratory Journal, 56(4), 2000117.
Hoffmann, M., Kleine-Weber, H., Schroeder, S., Krüger, N., Herrler, T., Erichsen, S., & Pöhlmann, S. (2020). SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell, 181(2), 271–280.
Chen, L., Li, Q., Zheng, D., Jiang, H., Wei, Y., Zou, L., Feng, L., Xiong, G., Sun, G., Wang, H., Zhao, Y., & Qiao, J. (2020). Clinical characteristics of pregnant women with Covid-19 in Wuhan, China. New England Journal of Medicine, 382(25), e100.
Calleja, D. J., Lessene, G., & Komander, D. (2022). Inhibitors of SARS-CoV-2 PLpro. Frontiers in Chemistry, 10, 876212.
Kumar, A., Singh, R., Kaur, J., Pandey, S., Sharma, V., Thakur, L., Sati, S., Mani, S., Asthana, S., Sharma, T. K., Chaudhuri, S., Bhattacharyya, S., & Kumar, N. Wuhan to world: The COVID-19 pandemic. Frontiers in Cellular and Infection Microbiology, (11), 596201.
Pietrobelli, A., Pecoraro, L., Ferruzzi, A., Heo, M., Faith, M., Zoller, T., Antoniazzi, F., Piacentini, G., Fearnbach, S. N., & Heymsfield, S. B. (2020). Effects of COVID-19 lockdown on lifestyle behaviors in children with obesity living in Verona, Italy: A longitudinal study. Obesity (Silver Spring), 28(8), 1382–1385.
Jeon, S., Ko, M., Lee, J., Choi, I., Byun, S. Y., Park, S., Shum, D., & Kim, S. (2020). Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs. Antimicrobial Agents and Chemotherapy, 64(7), e00819–e00820.
Annweiler, C., Hanotte, B., Grandin de, C., Sabatier, J. M., Lafaie, L., & Célarier, T. (2020). Vitamin D and survival in COVID-19 patients: A quasi-experimental study. The Journal of Steroid Biochemistry and Molecular Biology, 204, 105771.
Nieto-Torres, J. L., Verdiá-Báguena, C., Jimenez-Guardeño, J. M., Regla-Nava, J. A., Castaño-Rodriguez, C., Fernandez-Delgado, R., Torres, J., Aguilella, V. M., & Enjuanes, L. (2015). Severe acute respiratory syndrome coronavirus E protein transports calcium ions and activates the NLRP3 inflammation. Virology, 9(485), 330–339.
Guo, Y. R., Cao, Q. D., Hong, Z. S., Tan, Y. Y., Chen, S. D., Jin, H. J., Tan, K. S., Wang, D. Y., & Yan, Y. (2019). The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – an update on the status. Military Medical Research, 7(1), 11.
Casanova, L. M., Jeon, S., Rutala, W. A., Weber, D. J., & Sobsey, M. D. (2010). Effects of air temperature and relative humidity on coronavirus survival on surfaces. Applied and Environmental Microbiology, 76(9), 2712–2717.
Jean, S. S., Lee, P. I., & Hsueh, P. R. (2020). Treatment options for COVID-19: The reality and challenges. Journal of Microbiology, Immunology and Infection, 53(3), 436–443.
Bilinska, K., Jakubowska, P., Von Bartheld, C. S., & Butowt, R. (2020). Expression of the SARS-CoV-2 entry proteins, ACE2 and TMPRSS2, in cells of the olfactory epithelium: Identification of cell types and trends with age. ACS Chemical Neuroscience, 11(11), 1555–1562.
Xie M and Chen Q. (2020) Insight into 2019 novel coronavirus – An updated interim review and lessons from SARS-CoV and MERS-CoV International Journal of Infectious Diseases, 94, 119–124.
Fakhri, S., Piri, S., Majnooni, M. B., Farzaei, M. H., & Echeverría, J. (2021). Targeting neurological manifestations of coronaviruses by candidate phytochemicals: A mechanistic approach. Front Pharmacology, 11, 621099.
Bojkova, D., Klann, K., Koch, B., Widera, M., Krause, D., Ciesek, S., Cinatl, J., & Münch, C. (2020). Proteomics of SARS-CoV-2-infected host cells reveals therapy targets. Nature, 583(7816), 469–472.
Hillen, H. S., Kokic, G., Farnung, L., Dienemann, C., Tegunov, D., & Cramer, P. (2020). Structure of replicating SARS-CoV-2 polymerase. Nature, 584(7819), 154–156.
Te Velthuis AJ, van den Worm SH, Snijder EJ. (2020) The SARS-coronavirus nsp7+nsp8 complex is a unique multimeric RNA polymerase capable of both de novo initiation and primer extension. Nucleic Acids Research. 40 (4), 1737-1747. Doi: https://doi.org/10.1093/nar/gkr893. Epub 2011 Oct 29. PMID: 22039154; PMCID: PMC3287201.
Self, W. H., Semler, M. W., Leither, L. M., et al. (2020). Effect of hydroxychloroquine on clinical status at 14 days in hospitalized patients with COVID-19: A randomized clinical trial. JAMA, 324(21), 2165–2176.
Athmer, J., Fehr, A. R., Grunewald, M., Smith, E. C., Denison, M. R., & Perlman, S. (2017). In situ tagged nsp15 reveals interactions with coronavirus replication/transcription complex-associated proteins. mBio, 8(1), e02320–e02316.
Boopathi, S., Poma, A. B., & Kolandaivel, P. (2021). Novel 2019 coronavirus structure, mechanism of action, antiviral drug promises and rule out against its treatment. Journal of Biomolecular Structure and Dynamics, 39(9), 3409–3418.
Li, F. (2016). Structure, function, and evolution of coronavirus spike proteins. Annual Review of Virology, 3(1), 237–261.
Mirza, M. U., & Froeyen, M. (2020). Structural elucidation of SARS-CoV-2 vital proteins: Computational methods reveal potential drug candidates against main protease, Nsp12 polymerase and Nsp13 helicase. Journal of Pharmaceutical Analysis, 10(4), 320–328.
Costela-Ruiz, V. J., Illescas-Montes, R., Puerta-Puerta, J. M., Ruiz, C., & Melguizo-Rodríguez, L. (2020). SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine and Growth Factor Review, 54, 62–75.
Ulhaq, Z. S., & Soraya, G. V. (2020). Interleukin-6 as a potential biomarker of COVID-19 progression. Medecine et Maladies Infectieuses, 50(4), 382–383.
Gui, M., Song, W., Zhou, H., Xu, J., Chen, S., Xiang, Y., & Wang, X. (2017). Cryo-electron microscopy structures of the SARS-CoV spike glycoprotein reveal a prerequisite conformational state for receptor binding. Cell Research, 27(1), 119–129.
Chivese, T., Musa, O. A. H., Hindy, G., Al-Wattary, N., Badran, S., Soliman, N., Aboughalia, A. T. M., Matizanadzo, J. T., Emara, M. M., Thalib, L., & Doi, S. A. R. (2021). Efficacy of chloroquine and hydroxychloroquine in treating COVID-19 infection: A meta-review of systematic reviews and an updated meta-analysis. Travel Medicine and Infectious Disease, 43, 102135.
Alhumaid, S., Al Mutair, A., Al Alawi, Z., Alhmeed, N., Zaidi, A. R. Z., & Tobaiqy, M. (2020). Efficacy and safety of Lopinavir/Ritonavir for treatment of COVID-19: A systematic review and meta-analysis. Tropical Medicine and Infectious Disease, 5, 180.
Sanders, J. M., Monogue, M. L., Jodlowski, T. Z., & Cutrell, J. B. (2020). Pharmacologic treatments for coronavirus disease 2019 (COVID-19): A review. JAMA, 323(18), 1824–1836.
McKee, D. L., Sternberg, A., Stange, U., Laufer, S., & Naujokat, C. (2020). Candidate drugs against SARS-CoV-2 and COVID-19. Pharmacological Research, 157, 104859.
Yang, L., Liu, W., Yu, X., Wu, M., Reichert, J. M., & Ho, M. (2020). COVID-19 antibody therapeutics tracker: A global online database of antibody therapeutics for the prevention and treatment of COVID-19. Antibody Therapeutics, 3(3), 205–212.
Amin Jafaria, A., & Ghasemi, S. (2020). The possible of immunotherapy for COVID-19: A systematic review. International Immunopharmacology, 83, 106455.
Cong, X., Wu, J., Vittner, D., Xu, W., Hussain, N., Galvin, S., Fitzsimons, M., McGrath, J. M., & Henderson, W. A. (2017). The impact of cumulative pain/stress on neurobehavioral development of preterm infants in the NICU. Early Human Development, 108, 9–16.
Beigel, J. H. (2021). What is the role of remdesivir in patients with COVID-19? Current Opinion in Critical Care, 27(5), 487–492.
Colson, P., Devaux, C. A., Lagier, J.-C., Gautret, P., & Raoult, D. (2021). A possible role of remdesivir and plasma therapy in the selective sweep and emergence of new SARS-CoV-2 variants. Journal of Clinical Medicine., 10(15), 3276.
Maggio, R., & Corsini, G. U. (2020). Repurposing the mucolytic cough suppressant and TMPRSS2 protease inhibitor bromhexine for the prevention and management of SARS-CoV-2 infection. Pharmacological Research, 157, 104837.
Iwata-Yoshikawa, N., Okamura, T., Shimizu, Y., Hasegawa, H., Takeda, M., & Nagata, N. (2019). TMPRSS2 contributes to virus spread and immunopathology in the airways of murine models after coronavirus infection. Journal of Virology, 93(6), e01815–e01818.
Bein, B., Bachmann, M., Huggett, S., & Wegermann, P. SARS-CoV-2/COVID-19: Empfehlungen zu Diagnostik und Therapie [SARS CoV-2/COVID-19: Evidence-based recommendation on diagnosis and therapy]. Anasthesiol Intensivmed Notfallmed Schmerzther, 55(4), 257–265.
Alosaimi, B., Hamed, M. E., Naeem, A., Alsharef, A. A., AlQahtani, S. Y., AlDosari, K. M., Alamri, A. A., Al-Eisa, K., Khojah, T., Assiri, A. M., & Enani, M. A. (2020). MERS-CoV infection is associated with downregulation of genes encoding Th1 and Th2 cytokines/chemokines and elevated inflammatory innate immune response in the lower respiratory tract. Cytokine, 126, 154895.
Conti, P., Ronconi, G., Caraffa, A., Gallenga, C. E., Ross, R., Frydas, I., & Kritas, S. K. (2020). Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): Anti-inflammatory strategies. Journal of Biological Regulators and Homeostatic Agents, 34(2), 327–331.
Magro, C., Mulvey, J. J., Berlin, D., Nuovo, G., Salvatore, S., Harp, J., Baxter-Stoltzfus, A., & Laurence, J. (2020). Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Translational Research, 220, 1–13.
Sinha, N., & Balayla, G. (2020). Hydroxychloroquine and COVID-19. Postgraduate Medical Journal, 96, 550–555.
Dauby, N., & Bottieau, E. (2021). The unfinished story of hydroxychloroquine in COVID-19: The right anti-inflammatory dose at the right moment? International Journal of Infectious Diseases, 103, 1–2.
Gralinski, L. E., Sheahan, T. P., Morrison, T. E., Menachery, V. D., Jensen, K., Leist, S. R., Whitmore, A., Heise, M. T., & Baric, R. S. (2018). Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. mBio, 9(5), e01753–e01718.
Ahmed, M. H., & Hassan, A. (2020). Dexamethasone for the treatment of coronavirus disease (COVID-19): A review. SN Comprehensive Clinical Medicine, 2, 2637–2646.
Lester, M., Sahin, A., & Pasyar, A. (2020). The use of dexamethasone in the treatment of COVID-19. Ann Med Surg (Lond)., 56, 218–219.
Schett, G., Sticherling, M., & Neurath, M. F. (2020). COVID-19: Risk for cytokine targeting in chronic inflammatory diseases? Nature Reviews Immunology, 20, 271–272.
Khan, F. A., Stewart, I., Fabbri, L., et al. (2021). Systematic review and meta-analysis of anakinra, sarilumab, siltuximab and tocilizumab for COVID-19. Thorax, 76, 907–919.
Seneviratne, S. L., Abeysuriya, V., Mel, S., Zoysa, I., & Niloofa, R. (2020). Favipiravir in Covid-19. International Journal of Progressive Sciences and Technologies, 19, 143–145.
Pilkington, V., Pepperrell, T., & Hill, A. (2020). A review of the safety of favipiravir – A potential treatment in the COVID-19 pandemic? Journal of Virus Eradication, 6(2), 45–51.
Rizk, J. G., Kalantar-Zadeh, K., Mehra, M. R., et al. (2020). Pharmaco-immunomodulatory therapy in COVID-19. Drugs, 80, 1267–1292.
Gottlieb, R. L., Lang, F. M., Criner, G. J., Mathews, K. S., Wang, T. S., Rice, T. W., Madduri, D., & Lowry, S. Anti-granulocyte–macrophage colony–stimulating factor monoclonal antibody gimsilumab for COVID-19 pneumonia: a randomized, double-blind, Placebo-controlled Trial. American Journal of Respiratory and Critical Care Medicine, 205(11), 1290–1299.
King, R. G., Silva-Sanchez, A., Peel, J. N., Botta, D., Dickson, A. M., Pinto, A. K., Meza-Perez, S., Allie, S. R., Schultz, M. D., Liu, M., & Bradley, J. E. (2021). Single-dose intranasal administration of AdCOVID elicits systemic and mucosal immunity against SARS-CoV-2 and fully protects mice from lethal challenge. Vaccines, 9(8), 881.
Arroyo, R., Grant, S. N., Colombo, M., Salvioni, L., Corsi, F., Truffi, M., Ottolina, D., Hurst, B., Salzberg, M., Prosperi, D., & Kingma, P. S. (2021). Full-length recombinant hSP-D binds and inhibits SARS-CoV-2. Biomolecules, 11(8), 1114.
Philippidis, A. (2020). COVID-19: Top 60 drug treatments in development: The biopharma industry is ramping up the development of dozens of potential drug therapies and clinical testing in an all-hands effort to combat the pandemic. Genetic Engineering & Biotechnology News, 40(4), 10–13.
Basha, S. H. (2020). Corona virus drugs–A brief overview of past, present and future. Journal of PeerScientist, 2(2), e1000013.
Sareen, K., Bose, R., Singh, R., & Boddu, L. (2020). Treatment of COVID-19. Praxis Undergraduate Medical Research Journal, 1, 3.
Sehgal, D. (2020). Analysis of vaccines to tackle Covid-19 with patent. The Pharma Innovation Journal, 9(7), 498–513.
Bechthold, E., Schreiber, J. A., Lehmkuhl, K., Frehland, B., Schepmann, D., Bernal, F. A., Daniliuc, C., Álvarez, I., Garcia, C. V., Schmidt, T. J., & Seebohm, G. (2020). Ifenprodil stereoisomers: Synthesis, absolute configuration, and correlation with biological activity. Journal of Medicinal Chemistry, 64(2), 1170–1179.
Shoemaker, R. H., Panettieri, R. A., Jr., Libutti, S. K., Hochster, H. S., Watts, N. R., Wingfield, P. T., Starkl, P., Pimenov, L., Gawish, R., Hladik, A., & Knapp, S. (2022). Development of an aerosol intervention for COVID-19 disease: Tolerability of soluble ACE2 (APN01) administered via nebulizer. PloS one, 17(7), e0271066.
Zoufaly, A., Poglitsch, M., Aberle, J. H., Hoepler, W., Seitz, T., Traugott, M., Grieb, A., Pawelka, E., Laferl, H., Wenisch, C., & Neuhold, S. (2020). Human recombinant soluble ACE2 in severe COVID-19. The Lancet Respiratory Medicine, 8(11), 1154–1158.
Bakovic, A., Risner, K., Bhalla, N., Alem, F., Chang, T. L., Weston, W. K., Harness, J. A., & Narayanan, A. (2021). Brilacidin demonstrates inhibition of SARS-CoV-2 in cell culture. Viruses, 13(2), 271.
Trasino, S. E. (2020). A role for retinoids in the treatment of COVID-19? Clinical and Experimental Pharmacology and Physiology, 47(10), 1765–1767.
Meini, S., Pagotto, A., Longo, B., Vendramin, I., Pecori, D., & Tascini, C. (2020). Role of Lopinavir/Ritonavir in the treatment of Covid-19: A review of current evidence, guideline recommendations, and perspectives. Journal of Clinical Medicine, 9(7), 2050.
Khalili, J. S., Zhu, H., Mak, N. S., Yan, Y., & Zhu, Y. (2020). Novel coronavirus treatment with ribavirin: Groundwork for an evaluation concerning COVID-19. Journal of Medical Virology, 92(7), 740–746.
Cortegiani, A., Ingoglia, G., Ippolito, M., Giarratano, A., & Einav, S. (2020). A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. Journal of Critical Care, 57, 279–283.
Nojomi, M., Yassin, Z., Keyvani, H., Makiani, M. J., Roham, M., Laali, A., Dehghan, N., Navaei, M., & Ranjbar, M. (2020). Effect of Arbidol (Umifenovir) on COVID-19: A randomized controlled trial. BMC Infectious Diseases, 20(1), 1–0.
Funding
As this is a review paper, no funding was required.
Author information
Authors and Affiliations
Contributions
All the authors contributed equally.
Corresponding author
Ethics declarations
Consent for publication
The authors give the consent for publication.
Conflict of interest
The authors do not have any conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Talukder, P., Saha, A., Roy, S. et al. Drugs for COVID-19 Treatment: A New Challenge. Appl Biochem Biotechnol 195, 3653–3670 (2023). https://doi.org/10.1007/s12010-023-04439-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04439-4