Abstract
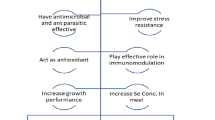
Feeds for aquaculture animals are designed to provide them with the greatest amount of nourishment they need to carry out their regular physiological activities, such as maintaining a potent natural immune system and boosting growth and reproduction. However, the problems that severely hamper this sector's ability to contribute to achieving global food security include disease prevalence, chemical pollution, environmental deterioration, and inadequate feed usage. The regulated release of active aquafeed components; limited water solubility, bioaccessibility, and bioavailability, as well as their potent odour and flavour, limit their utilisation. They are unstable under high temperatures, acidic pH, oxygen, or light. Recent advancements in nano-feed for aquaculture (fish/shrimp) have attract enormous attention due to its excellent nutritional value, defeating susceptibility and perishability. Encapsulation is a multifunctional smart system that could bring benefits of personalized medicine; minimize costs and resources in the preclinical and clinical study in pharmacology. It guarantees the coating of the active ingredient as well as its controlled release and targeted distribution to a particular area of the digestive tract. For instance, using nanotechnology to provide more effective fish/shrimps feed for aquaculture species. The review enables a perspective points on safety and awareness in aquafeeds that have been made by the advancements of nanosystem. Therefore, potential of nano-delivery system in aquafeed industry for aquaculture act as concluding remark on future directions.





Similar content being viewed by others
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Gabriel, N. N., Habte-Tsion, H. M., & Haulofu, M. (2022). Perspectives of nanotechnology in aquaculture: Fish nutrition, disease, and water treatment. In A. Krishnan, B. Ravindran, B. Balasubramanian, H. C. Swart, S. J. Panchu, & R. Prasad (Eds.), Emerging nanomaterials for advanced technologies (pp. 463–485). Springer International Publishing. https://doi.org/10.1007/978-3-030-80371-1_15
Albrecht, M. A., Evans, C. W., & Raston, C. L. (2006). Green chemistry and the health implications of nanoparticles. Green Chemistry, 8(5), 417–432. https://doi.org/10.1039/b517131h
Lall, S. P., & Tibbetts, S. M. (2009). Nutrition, feeding, and behavior of fish veterinary clinics of North America. Exotic Animal Practice, 12(2), 361–372. https://doi.org/10.1016/j.cvex.2009.01.005
Krishnani, K. K. (2021). Finfish-based bioaugmentation technology for enhancing Shrimp Grower’s income and livelihood. Agricultural Research, 10(3), 361–368. https://doi.org/10.1007/s40003-020-00530-y
Wang, W., Sun, J., Liu, C., & Xue, Z. (2017). Application of immunostimulants in aquaculture: Current knowledge and future perspectives. Aquaculture Research, 48(1), 1–23. https://doi.org/10.1111/are.13161
Mourya, V. K., Inamdar, N., Nawale, R. B., & Kulthe, S. S. (2011). Polymeric micelles: General considerations and their applications. Indian J Pharm Educ Res, 45(2), 128–138.
Jafari, S. M. (2017). An overview of nanoencapsulation techniques and their classification. Nanoencapsulation Technologies for the Food and Nutraceutical Industries, 1–34. https://doi.org/10.1016/B978-0-12-809436-5.00001-X
Kothamasu, P., Kanumur, H., Ravur, N., Maddu, C., Parasuramrajam, R., & Thangavel, S. (2012). Nanocapsules: The weapons for novel drug delivery systems. BioImpacts, BI, 2(2), 71. https://doi.org/10.5681/bi.2012.011
Reza Mozafari, M., Johnson, C., Hatziantoniou, S., & Demetzos, C. (2008). Nanoliposomes and their applications in food nanotechnology. Journal of liposome research, 18(4), 309–327. https://doi.org/10.1080/08982100802465941
Bedford, M. A., & Schulze, H. (1998). Exogenous enzymes for pigs and poultry. Nutrition Research Reviews, 11(1), 91–114. https://doi.org/10.1079/NRR19980007
Soni, M., Maurya, A., Das, S., Prasad, J., Yadav, A., Singh, V. K., & Dwivedy, A. K. (2022). Nanoencapsulation strategies for improving nutritional functionality, safety and delivery of plant-based foods: Recent updates and future opportunities. Plant Nano Biology, 1, 100004. https://doi.org/10.1016/j.plana.2022.100004
da Cunha, J. A., de Ávila Scheeren, C., Fausto, V. P., de Melo, L. D. W., Henneman, B., Frizzo, C. P., & Baldisserotto, B. (2018). The antibacterial and physiological effects of pure and nanoencapsulated Origanummajorana essential oil on Fish infected with Aeromonashydrophila. Microbial pathogenesis, 124, 116–121. https://doi.org/10.1016/j.micpath.2018.08.040
Udo, I. U., Etukudo, U., & Anwana, U. I. U. (2018). Effects of chitosan and chitosan nanoparticles on water quality, growth performance, survival rate and meat quality of the African catfish, Clariasgariepinus. Nanoscience, 1(1), 12–25.https://doi.org/10.31058/j.nano.2018.11002
Navarro-Segura, L., Ros-Chumillas, M., López-Cánovas, A. E., García-Ayala, A., & López-Gómez, A. (2019). Nanoencapsulated essential oils embedded in ice improve the quality and shelf life of fresh whole seabream stored on ice. Heliyon, 5(6), e01804. https://doi.org/10.1016/j.heliyon.2019.e01804
Tom, A. P., Jayakumar, J. S., Biju, M., Somarajan, J., & Ibrahim, M. A. (2021). Aquaculture wastewater treatment technologies and their sustainability: A review. Energy Nexus, 4, 100022. https://doi.org/10.1016/j.nexus.2021.100022
Hedayati, S. A., Sheikh Veisi, R., Hosseini Shekarabi, S. P., ShahbaziNaserabad, S., Bagheri, D., & Ghafarifarsani, H. (2022). Effect of dietary Lactobacillus casei on physiometabolic responses and liver histopathology in common carp (Cyprinus carpio) after exposure to iron oxide nanoparticles. Biological trace element research, 200(7), 3346–3354. https://doi.org/10.1007/s12011-021-02906-9
Rajeshkumar, S., Venkatesan, C., Sarathi, M., Sarathbabu, V., Thomas, J., Basha, K. A., & Hameed, A. S. (2009). Oral delivery of DNA construct using chitosan nanoparticles to protect the shrimp from white spot syndrome virus (WSSV). Fish & Shellfish Immunology, 26(3), 429–437. https://doi.org/10.1016/j.fsi.2009.01.003
Strømme, M., Brohede, U., Atluri, R., & Garcia-Bennett, A. E. (2009). Mesoporous silica-based nanomaterials for drug delivery: Evaluation of structural properties associated with release rate. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology, 1(1), 140–148. https://doi.org/10.1002/wnan.13
Eldridge, J. H., Hammond, C. J., Meulbroek, J. A., Staas, J. K., Gilley, R. M., & Tice, T. R. (1990). Controlled vaccine release in the gut-associated lymphoid tissues. I. Orally administered biodegradable microspheres target the Peyer’s patches. Journal of Controlled Release, 11(1–3), 205–214. https://doi.org/10.1016/0168-3659(90)90133-E
Thiruvengadam, M., Rajakumar, G., & Chung, I. M. (2018). Nanotechnology: current uses and future applications in the food industry. 3 Biotech, 8(1), 1–13. https://doi.org/10.1007/s13205-018-1104-7
Ashouri, S., Keyvanshokooh, S., Salati, A. P., Johari, S. A., & Pasha-Zanoosi, H. (2015). Effects of different levels of dietary selenium nanoparticles on growth performance, muscle composition, blood biochemical profiles and antioxidant status of common carp (Cyprinus carpio). Aquaculture, 446, 25–29. https://doi.org/10.1016/j.aquaculture.2015.04.021
Stone, V., Nowack, B., Baun, A., van den Brink, N., von der Kammer, F., Dusinska, M., ..., & Fernandes, T. F. (2010). Nanomaterials for environmental studies: classification, reference material issues, and strategies for physico-chemical characterisation. Science of the Total Environment, 408(7), 1745–1754. https://doi.org/10.1016/j.scitotenv.2009.10.035
Bochtis, D. D., Sørensen, C. G., & Busato, P. (2014). Advances in agricultural machinery management: A review. Biosystems Engineering, 126, 69–81. https://doi.org/10.1016/j.biosystemseng.2014.07.012
Dong, H., Wang, P., Yang, Z., Li, R., Xu, X., & Shen, J. (2022). Dual improvement in curcumin encapsulation efficiency and lyophilized complex dispersibility through ultrasound regulation of curcumin–protein assembly. Ultrasonics Sonochemistry, 90, 106188. https://doi.org/10.1016/j.ultsonch.2022.106188
Albdour, S. A., Haddad, Z., Sharaf, O. Z., Alazzam, A., & Abu-Nada, E. (2022). Micro/nano-encapsulated phase-change materials (ePCMs) for solar photothermal absorption and storage: Fundamentals, recent advances, and future directions. Progress in Energy and Combustion Science, 93, 101037. https://doi.org/10.1016/j.pecs.2022.101037
Livney, Y. D. (2015). Nanostructured delivery systems in food: Latest developments and potential future directions. Current Opinion in Food Science, 3, 125–135. https://doi.org/10.1016/j.cofs.2015.06.010
Khezerlou, A., & Jafari, S. M. (2020). Nanoencapsulated bioactive components for active food packaging. In Handbook of food nanotechnology (pp. 493–532). Academic Press. https://doi.org/10.1016/B978-0-12-815866-1.00013-3
Bryant, S. J., Christofferson, A. J., Greaves, T. L., McConville, C. F., Bryant, G., & Elbourne, A. (2022). Bulk and interfacial nanostructure and properties in deep eutectic solvents: Current perspectives and future directions. Journal of Colloid and Interface Science, 608, 2430–2454. https://doi.org/10.1016/j.jcis.2021.10.163
Narasimhan, N. L., Bharath, R., Ramji, S. A., Tarun, M., & Arumugam, A. S. (2014). Numerical studies on the performance enhancement of an encapsulated thermal storage unit. International journal of thermal sciences, 84, 184–195. https://doi.org/10.1016/j.ijthermalsci.2014.05.003
Ravichandran, R. (2010). Nanotechnology applications in food and food processing: Innovative green approaches, opportunities and uncertainties for global market. International Journal of Green Nanotechnology: Physics and Chemistry, 1(2), P72–P96. https://doi.org/10.1080/19430871003684440
Murata, J. I., Ohya, Y., & Ouchi, T. (1997). Design of quaternary chitosan conjugate having antennary galactose residues as a gene delivery tool. Carbohydrate Polymers, 32(2), 105–109. https://doi.org/10.1016/S0144-8617(96)00154-3
Choi, M. J., Ruktanonchai, U., Min, S. G., Chun, J. Y., & Soottitantawat, A. (2010). Physical characteristics of fish oil encapsulated by β-cyclodextrin using an aggregation method or polycaprolactone using an emulsion–diffusion method. Food chemistry, 119(4), 1694–1703. https://doi.org/10.1016/j.foodchem.2009.09.052
Sanches-Fernandes, G. M., Sá-Correia, I., & Costa, R. (2022). Vibriosis outbreaks in aquaculture: Addressing environmental and public health concerns and preventive therapies using gilthead seabream farming as a model system. Frontiers in Microbiology, 13. https://doi.org/10.3389/fmicb.2022.904815
Rousta, L. K., Bodbodak, S., Nejatian, M., Yazdi, A. P. G., Rafiee, Z., Xiao, J., & Jafari, S. M. (2021). Use of encapsulation technology to enrich and fortify bakery, pasta, and cereal-based products. Trends in Food Science & Technology, 118, 688–710. https://doi.org/10.1016/j.tifs.2021.10.029
Kumar, S. R., Ahmed, V. I., Parameswaran, V., Sudhakaran, R., Babu, V. S., & Hameed, A. S. (2008). Potential use of chitosan nanoparticles for oral delivery of DNA vaccine in Asian sea bass (Lates calcarifer) to protect from Vibrio (Listonella) anguillarum. Fish & Shellfish Immunology, 25(1–2), 47–56. https://doi.org/10.1016/j.fsi.2007.12.004
Adomako, M., St-Hilaire, S., Zheng, Y., Eley, J., Marcum, R. D., Sealey, W., & Sheridan, P. P. (2012). Oral DNA vaccination of rainbow trout, Oncorhynchus mykiss (Walbaum), against infectious haematopoietic necrosis virus using PLGA [Poly (D, L-Lactic-Co-Glycolic Acid)] nanoparticles. Journal of Fish Diseases, 35(3), 203–214. https://doi.org/10.1111/j.1365-2761.2011.01338.x
Tian, J., & Yu, J. (2011). Poly (lactic-co-glycolic acid) nanoparticles as candidate DNA vaccine carrier for oral immunization of Japanese flounder (Paralichthysolivaceus) against lymphocystis disease virus. Fish & Shellfish Immunology, 30(1), 109–117. https://doi.org/10.1016/j.fsi.2010.09.016
Rezaei, A., Fathi, M., & Jafari, S. M. (2019). Nanoencapsulation of hydrophobic and low-soluble food bioactive compounds within different nanocarriers. Food Hydrocolloids, 88, 146–162. https://doi.org/10.1016/j.foodhyd.2018.10.003
Hamad, A. F., Han, J. H., Kim, B. C., & Rather, I. A. (2018). The intertwine of nanotechnology with the food industry. Saudi Journal of Biological Sciences, 25(1), 27–30. https://doi.org/10.1016/j.sjbs.2017.09.004
Maqsoudlou, A., Assadpour, E., Mohebodini, H., & Jafari, S. M. (2020). Improving the efficiency of natural antioxidant compounds via different nanocarriers. Advances in Colloid and Interface Science, 278, 102122. https://doi.org/10.1016/j.cis.2020.102122
Neo, Y. P., Ray, S., Jin, J., Gizdavic-Nikolaidis, M., Nieuwoudt, M. K., Liu, D., & Quek, S. Y. (2013). Encapsulation of food grade antioxidant in natural biopolymer by electrospinning technique: A physicochemical study based on zein–gallic acid system. Food Chemistry, 136(2), 1013–1021. https://doi.org/10.1016/j.foodchem.2012.09.010
Hassani, M., & Hasani, S. (2018). Nano-encapsulation of thyme essential oil in chitosan-Arabic gum system: evaluation of its antioxidant and antimicrobial properties. Trends in Phytochemical Research, 2(2), 75–82. https://doi.org/20.1001.1.25883623.2018.2.2.2.2
Bratovcic, A., & Suljagic, J. (2019). Micro-and nano-encapsulation in food industry. Croatian journal of Food Science and Technology, 11(1), 113–121. https://doi.org/10.17508/Cjfst.2019.11.1.17
Onwulata, C. I. (2013). Microencapsulation and functional bioactive foods. Journal of Food Processing and Preservation, 37(5), 510–532. https://doi.org/10.1111/j.1745-4549.2012.00680.x
Wang, T., Xue, J., Hu, Q., Zhou, M., & Luo, Y. (2017). Preparation of lipid nanoparticles with high loading capacity and exceptional gastrointestinal stability for potential oral delivery applications. Journal of colloid and interface science, 507, 119–130. https://doi.org/10.1016/j.jcis.2017.07.090
McClements, D. J. (2015). Encapsulation, protection, and release of hydrophilic active components: Potential and limitations of colloidal delivery systems. Advances in Colloid and Interface Science, 219, 27–53. https://doi.org/10.1016/j.cis.2015.02.002
Chávarri, M., Marañón, I., Ares, R., Ibáñez, F. C., Marzo, F., & del Carmen Villarán, M. (2010). Microencapsulation of a probiotic and prebiotic in alginate-chitosan capsules improves survival in simulated gastro-intestinal conditions. International Journal of Food Microbiology, 142(1–2), 185–189. https://doi.org/10.1016/j.ijfoodmicro.2010.06.022
Mohsenabadi, N., Rajaei, A., Tabatabaei, M., & Mohsenifar, A. (2018). Physical and antimicrobial properties of starch-carboxy methyl cellulose film containing rosemary essential oils encapsulated in chitosan nanogel. International Journal of Biological Macromolecules, 112, 148–155. https://doi.org/10.1016/j.ijbiomac.2018.01.034
Arroyo-Maya, I. J., & McClements, D. J. (2015). Biopolymer nanoparticles as potential delivery systems for anthocyanins: Fabrication and properties. Food Research International, 69, 1–8. https://doi.org/10.1016/j.foodres.2014.12.005
Trucillo, P., Campardelli, R., Aliakbarian, B., Perego, P., & Reverchon, E. (2018). Supercritical assisted process for the encapsulation of olive pomace extract into liposomes. The Journal of Supercritical Fluids, 135, 152–159. https://doi.org/10.1016/j.supflu.2018.01.018
Huang, Q., Yu, H., & Ru, Q. (2010). Bioavailability and delivery of nutraceuticals using nanotechnology. Journal of Food Science, 75(1), R50–R57. https://doi.org/10.1111/j.1750-3841.2009.01457.x
Pérez-Masiá, R., López-Nicolás, R., Periago, M. J., Ros, G., Lagaron, J. M., & López-Rubio, A. (2015). Encapsulation of folic acid in food hydrocolloids through nanospray drying and electrospraying for nutraceutical applications. Food Chemistry, 168, 124–133. https://doi.org/10.1016/j.foodchem.2014.07.051
Dasgupta, N., Ranjan, S., Mundra, S., Ramalingam, C., & Kumar, A. (2016). Fabrication of food grade vitamin-E nanoemulsion by low energy approach, characterization and its application. International Journal of Food Properties, 19(3), 700–708. https://doi.org/10.1080/10942912.2015.1042587
Rehman, A., Ahmad, T., Aadil, R. M., Spotti, M. J., Bakry, A. M., Khan, I. M., & Tong, Q. (2019). Pectin polymers as wall materials for the nano-encapsulation of bioactive compounds. Trends in Food Science & Technology, 90, 35–46. https://doi.org/10.1016/j.tifs.2019.05.015
Faiz, H., Zuberi, A., Nazir, S., Rauf, M., & Younus, N. (2015). Zinc oxide, zinc sulfate and zinc oxide nanoparticles as source of dietary zinc: comparative effects on growth and hematological indices of juvenile grass carp (Ctenopharyngodon idella). International Journal of Agriculture and Biology, 17(3), 568–574. https://doi.org/10.17957/IJAB/17.3.14.446
Raeisi, S., Ojagh, S. M., Quek, S. Y., Pourashouri, P., & Salaün, F. (2019). Nano-encapsulation of fish oil and garlic essential oil by a novel composition of wall material: Persian gum-chitosan. LWT, 116, 108494. https://doi.org/10.1016/j.lwt.2019.108494
Ricaurte, L., Correa, R. E. P., de Jesus Perea-Flores, M., & Quintanilla-Carvajal, M. X. (2017). Influence of milk whey on high-oleic palm oil nanoemulsions: Powder production, physical and release properties. Food Biophysics, 12(4), 439–450. https://doi.org/10.1007/s11483-017-9500-9
Bhushani, J. A., Kurrey, N. K., & Anandharamakrishnan, C. (2017). Nanoencapsulation of green tea catechins by electrospraying technique and its effect on controlled release and in-vitro permeability. Journal of Food Engineering, 199, 82–92. https://doi.org/10.1016/j.jfoodeng.2016.12.010
Pushpalatha, R., Selvamuthukumar, S., & Kilimozhi, D. (2018). Cross-linked, cyclodextrin-based nanosponges for curcumin delivery-Physicochemical characterization, drug release, stability and cytotoxicity. Journal of Drug Delivery Science and Technology, 45, 45–53. https://doi.org/10.1016/j.jddst.2018.03.004
Qian, K., Wu, J., Zhang, E., Zhang, Y., & Fu, A. (2015). Biodegradable double nanocapsule as a novel multifunctional carrier for drug delivery and cell imaging. International Journal of Nanomedicine, 10, 4149. https://doi.org/10.2147/IJN.S83731
Khosravi-Katuli, K., Prato, E., Lofrano, G., Guida, M., Vale, G., & Libralato, G. (2017). Effects of nanoparticles in species of aquaculture interest. Environmental Science and Pollution Research, 24(21), 17326–17346. https://doi.org/10.1007/s11356-017-9360-3
Lall, S. P., & Dumas, A. (2022). Nutritional requirements of cultured fish: Formulating nutritionally adequate feeds. In D. A. Davis (Ed.), Feed and feeding practices in aquaculture, 2nd edn. (pp. 65–132). Woodhead Publishing. https://doi.org/10.1016/B978-0-12-821598-2.00005-9
Chen, H., & Yada, R. (2011). Nanotechnologies in agriculture: New tools for sustainable development. Trends in Food Science & Technology, 22(11), 585–594. https://doi.org/10.1016/j.tifs.2011.09.004
Can, E., Kizak, V., Kayim, M., Can, S. S., Kutlu, B., Ates, M., & Demirtas, N. (2011). Nanotechnological applications in aquaculture-seafood industries and adverse effects of nanoparticles on environment. Journal of Materials Science and Engineering, 5, 605–609.
Peng, D., Zhang, J., Liu, Q., & Taylor, E. W. (2007). Size effect of elemental selenium nanoparticles (Nano-Se) at supranutritional levels on selenium accumulation and glutathione S-transferase activity. Journal of inorganic biochemistry, 101(10), 1457–1463. https://doi.org/10.1016/j.jinorgbio.2007.06.021
Sarkar, B., Mahanty, A., Gupta, S. K., Choudhury, A. R., Daware, A., & Bhattacharjee, S. (2022). Nanotechnology: A next-generation tool for sustainable aquaculture. Aquaculture, 546, 737330. https://doi.org/10.1016/j.aquaculture.2021.737330
Abdel-Warith, A. W. A., El-Bab, A. F. F., Younis, E. S. M., Al-Asgah, N. A., Allam, H. Y., Abd-Elghany, M. F., & Shamlol, F. S. (2020). Using of chitosan nanoparticles (CsNPs), Spirulina as a feed additives under intensive culture system for black tiger shrimp (Penaeus monodon). Journal of King Saud University-Science, 32(8), 3359–3363. https://doi.org/10.1016/j.jksus.2020.09.022
Sáez, M. I., Vizcaíno, A. J., Alarcón, F. J., & Martínez, T. F. (2018). Feed pellets containing chitosan nanoparticles as plasmid DNA oral delivery system for Fish: In vivo assessment in gilthead sea bream (Sparus aurata) juveniles. Fish & Shellfish Immunology, 80, 458–466. https://doi.org/10.1016/j.fsi.2018.05.055
Sarkar, B., Bhattacharjee, S., Daware, A., Tribedi, P., Krishnani, K. K., & Minhas, P. S. (2015). Selenium nanoparticles for stress-resilient fish and livestock. Nanoscale Research Letters, 10, 371. https://doi.org/10.1186/s11671-015-1073-2
Bakhshayesh, S., Seifdavati, J., Seifzadeh, S., Mirzaei Aghjeh Gheshlagh, F., Abdi Benmar, H., & Vahedi, V. (2018). The effect of in Ovo Injection of Nanoparticles of Zinc Oxide on Hatching, Growth Performance and Carcass Yield of Broiler Chicks. Research On Animal Production (Scientific and Research), 9(21), 86–92. https://doi.org/10.29252/rap.9.21.86
Vimal, S., Taju, G., Nambi, K. N., Majeed, S. A., Babu, V. S., Ravi, M., & Hameed, A. S. (2012). Synthesis and characterization of CS/TPP nanoparticles for oral delivery of gene in Fish. Aquaculture, 358, 14–22. https://doi.org/10.1016/j.aquaculture.2012.06.012
Bhoopathy, S., Inbakandan, D., Rajendran, T., Chandrasekaran, K., Reddy, B. A., Kasilingam, R., & Dharani, G. (2021). Dietary supplementation of curcumin-loaded chitosan nanoparticles stimulates immune response in the white leg shrimp Litopenaeus vannamei challenged with Vibrio harveyi. Fish & Shellfish Immunology, 117, 188–191. https://doi.org/10.1016/j.fsi.2021.08.002
Bhoopathy, S., Inbakandan, D., Rajendran, T., Chandrasekaran, K., Kasilingam, R., &Gopal, D. (2021). Curcumin loaded chitosan nanoparticles fortify shrimp feed pellets with enhanced antioxidant activity. Materials Science and Engineering: C, 120, 111737. https://doi.org/10.1016/j.msec.2020.111737.
Saravanadevi, K., Devi, N. R., Dorothy, R., Joany, R. M., Rajendran, S., & Nguyen, T. A. (2022). Nanotechnology for agriculture: an introduction. In Nanosensors for Smart Agriculture (pp. 3–23). Elsevier. https://doi.org/10.1016/B978-0-12-824554-5.00013-6.
Tello-Olea, M., Rosales-Mendoza, S., Campa-Córdova, A. I., Palestino, G., Luna-González, A., Reyes-Becerril, M., & Angulo, C. (2019). Gold nanoparticles (AuNP) exert immunostimulatory and protective effects in shrimp (Litopenaeus vannamei) against Vibrio parahaemolyticus. Fish & shellfish immunology, 84, 756–767. https://doi.org/10.1016/j.fsi.2018.10.056
Bunglavan, S. J., Garg, A. K., Dass, R. S., & Shrivastava, S. (2014). Use of nanoparticles as feed additives to improve digestion and absorption in livestock. Livest Res Int, 2(3), 36–47.
Shah, B. R., & Mraz, J. (2020). Advances in nanotechnology for sustainable aquaculture and fisheries. Reviews in Aquaculture, 12(2), 925–942. https://doi.org/10.1111/raq.12356
Krishnani, K. K., Boddu, V. M., Chadha, N. K., Chakraborty, P., Kumar, J., Krishna, G., & Pathak, H. (2022). Nanostructured materials from plant, animal, and fisheries wastes: Potential and valorization for application. Agriculture, 1–36. https://doi.org/10.21203/rs.3.rs-1844329/v1
Ojagh, S. M., Rezaei, M., Razavi, S. H., & Hosseini, S. M. H. (2010). Effect of chitosan coatings enriched with cinnamon oil on the quality of refrigerated rainbow trout. Food Chemistry, 120(1), 193–198. https://doi.org/10.1016/j.foodchem.2009.10.006
Najafi, Z., Cetinkaya, T., Bildik, F., Altay, F., & Yeşilçubuk, N. Ş. (2022). Nanoencapsulation of saffron (Crocus sativus L.) extract in zein nanofibers and their application for the preservation of sea bass fillets. LWT, 163, 113588. https://doi.org/10.1016/j.lwt.2022.113588
Rao, M. A. (2009). Nanoscale particles in food and food packaging. Journal of Food Science, 74(9). https://doi.org/10.1111/j.1750-3841.2009.01420.x.
Das, M., Saxena, N., & Dwivedi, P. D. (2009). Emerging trends of nanoparticles application in food technology: Safety paradigms. Nanotoxicology, 3(1), 10–18. https://doi.org/10.1080/17435390802504237
Martirosyan, A., & Schneider, Y. J. (2014). Engineered nanomaterials in food: Implications for food safety and consumer health. International Journal of Environmental Research and Public Health, 11(6), 5720–5750. https://doi.org/10.3390/ijerph110605720
Sozer, N., & Kokini, J. L. (2009). Nanotechnology and its applications in the food sector. Trends in Biotechnology, 27(2), 82–89. https://doi.org/10.1016/j.tibtech.2008.10.010
Kang, S., Pinault, M., Pfefferle, L. D., & Elimelech, M. (2007). Single-walled carbon nanotubes exhibit strong antimicrobial activity. Langmuir, 23(17), 8670–8673. https://doi.org/10.1021/la701067r
Chau, C. F., Wu, S. H., & Yen, G. C. (2007). The development of regulations for food nanotechnology. Trends in Food Science & Technology, 18(5), 269–280. https://doi.org/10.1016/j.tifs.2007.01.007
Perez, R., & Gaonkar, A. G. (2014). Commercial applications of microencapsulation and controlled delivery in food and beverage products. in microencapsulation in the food industry (pp. 543–549). Academic Press. https://doi.org/10.1016/B978-0-12-404568-2.00041-8.
Ross, S. A., Srinivas, P. R., Clifford, A. J., Lee, S. C., Philbert, M. A., & Hettich, R. L. (2004). New technologies for nutrition research. The Journal of Nutrition, 134(3), 681–685. https://doi.org/10.1093/jn/134.3.681
Shrivastava, S., & Dash, D. (2009). Agrifood nanotechnology: a tiny revolution in food and agriculture. Journal of Nano Research, 6, 1–14. https://doi.org/10.4028/www.scientific.net/JNanoR.6.1
Augustin, M. A., & Sanguansri, P. (2009). Nanostructured materials in the food industry. Advances in Food and Nutrition Research, 58, 183–213. https://doi.org/10.1016/S1043-4526(09)58005-9
Farhang, B. (2009). Nanotechnology and applications in food safety. In Global issues in food science and technology (pp. 401–410). Academic Press. https://doi.org/10.1016/B978-0-12-374124-0.00022-3
Wang, D., Wang, K., Zhao, L., Liu, X., & Hu, Z. (2023). Fabrication and application of pickering emulsion stabilized by high pressure homogenization modified longan shell nanofiber. Journal of Food Engineering, 339, 111264. https://doi.org/10.1016/j.jfoodeng.2022.111264
Li, X., Lenhart, J. J., & Walker, H. W. (2012). Aggregation kinetics and dissolution of coated silver nanoparticles. Langmuir, 28(2), 1095–1104. https://doi.org/10.1021/la202328n
Xiu, Z. M., Ma, J., & Alvarez, P. J. (2011). Differential effect of common ligands and molecular oxygen on antimicrobial activity of silver nanoparticles versus silver ions. Environmental Science & Technology, 45(20), 9003–9008. https://doi.org/10.1021/es201918f
Wang, H., Burgess, R. M., Cantwell, M. G., Portis, L. M., Perron, M. M., Wu, F., & Ho, K. T. (2014). Stability and aggregation of silver and titanium dioxide nanoparticles in seawater: Role of salinity and dissolved organic carbon. Environmental Toxicology and Chemistry, 33(5), 1023–1029. https://doi.org/10.1002/etc.2529
SalariJoo, H., Kalbassi, M. R., & Johari, S. A. (2012). Effect of water salinity on acute toxicity of colloidal silver nanoparticles in rainbow trout (Oncorhynchus mykiss) larvae. Iranian Journal of Health and Environment, 5(2), 121–131.
Magesky, A., & Pelletier, É. (2018). Cytotoxicity and physiological effects of silver nanoparticles on marine invertebrates. In Q. Saquib, M. Faisal, A. Al-Khedhairy, & A. Alatar (Eds.), Cellular and molecular toxicology of nanoparticles. Advances in experimental medicine and biology, 1048. Springer. https://doi.org/10.1007/978-3-319-72041-8_17
Kruszewski, M., Brzoska, K., Brunborg, G., Asare, N., Dobrzyńska, M., Dušinská, M., & Refsnes, M. (2011). Toxicity of silver nanomaterials in higher eukaryotes. Advances in Molecular Toxicology, 5, 179–218. https://doi.org/10.1016/B978-0-444-53864-2.00005-0
Luis, A. I., Campos, E. V., Oliveira, J. L., Vallim, J. H., Proença, P. L., Castanha, R. F., & Fraceto, L. F. (2021). Ecotoxicity evaluation of polymeric nanoparticles loaded with ascorbic acid for fish nutrition in aquaculture. Journal of Nanobiotechnology, 19(1), 1–22. https://doi.org/10.1186/s12951-021-00910-8
Mishra, P., Kumar, R. S. S., Jerobin, J., Thomas, J., Mukherjee, A., & Chandrasekaran, N. (2014). Study on antimicrobial potential of neem oil nanoemulsion against P seudomonas aeruginosa infection in L abeo rohita. Biotechnology and Applied Biochemistry, 61(5), 611–619. https://doi.org/10.1002/bab.1213
Valentim, D. S. S., Duarte, J. L., Oliveira, A. E. M. F. M., Cruz, R. A. S., Carvalho, J. C. T., Solans, C., & Tavares-Dias, M. (2018). Effects of a nanoemulsion with Copaifera officinalis oleoresin against monogenean parasites of Colossoma macropomum: A Neotropical Serrasalmidae. Journal of fish Diseases, 41(7), 1041–1048. https://doi.org/10.1111/jfd.12793
Swathy, J. S., Mishra, P., Thomas, J., Mukherjee, A., & Chandrasekaran, N. (2018). Antimicrobial potency of high-energy emulsified black pepper oil nanoemulsion against aquaculture pathogen. Aquaculture, 491(210), 220. https://doi.org/10.1016/j.aquaculture.2018.03.045
Acknowledgements
We thanks to B. S. Abdur Rahman Crescent Institute of Science and Technology to be gratefully acknowledged for facilitating this study.
Funding
This research was supported by the Indian Council of Medical Research [Project ID: 2020–4964].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahmed, J., Vasagam, K. & Ramalingam, K. Nanoencapsulated Aquafeeds and Current Uses in Fisheries/Shrimps: A Review. Appl Biochem Biotechnol 195, 7110–7131 (2023). https://doi.org/10.1007/s12010-023-04418-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04418-9