Abstract
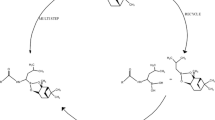
Bordetella pertussis, the causative agent of whooping cough, is an opportunistic virulent bacterial pathogen that is resistant to a wide range of antibiotics due to a variety of resistance mechanisms. Looking at the increasing number of infections caused by B. pertussis and its resistance to diverse antibiotics, it is essential to develop alternative strategies to fight against B. pertussis. Diaminopimelate epimerase (DapF) is an important enzyme of the lysine biosynthesis pathway in B. pertussis that catalyzes the formation of meso-2, 6-diaminoheptanedioate (meso-DAP), which is an important step in lysine metabolism. Therefore, Bordetella pertussis diaminopimelate epimerase (DapF) becomes an ideal target for antimicrobial drug development. In the present study, computational modelling, functional characterization, binding studies, and docking studies of BpDapF with lead compounds were carried out using different in silico tools. In silico prediction results in the secondary structure, 3-D structure analysis, and protein-protein interaction analysis of BpDapF. Docking studies further showed the respective amino acid residues for ligands in the phosphate‑binding loop of BpDapF play a vital role in the formation of H‑bonds with these ligands. The site where the ligand was bound is a deep groove, which is regarded as the binding cavity of the protein. Biochemical studies indicated that Limonin (binding energy − 8.8 kcal/mol), Ajmalicine (binding energy − 8.7 kcal/mol), Clinafloxacin (binding energy − 8.3 kcal/mol), Dexamethasone (binding energy − 8.2 kcal/mol), and Tetracycline (binding energy − 8.1 kcal/mol) exhibited promising binding towards the drug target DapF of B. pertussis in comparison with the binding between other drugs and act as the potential inhibitors of BpDapF that eventually can reduce the catalytic activity of BpDapF.








Similar content being viewed by others
Data Availability
Not applicable.
References
Hor, L., Dobson, R. C. J., Downton, M. T., Wagner, J., Hutton, C. A., & Perugini, M. A. (2013). Dimerization of bacterial diaminopimelate epimerase is essential for catalysis. Journal of Biological Chemistry, 288, 9238–9248.
Hutton, C., Southwood, T., & Turner, J. (2003). Inhibitors of lysine biosynthesis as antibacterial agents. Mini-Reviews in Medicinal Chemistry, 3, 115–127.
Hutton, C. A., Perugini, M. A., & Gerrard, J. A. (2007). Inhibition of lysine biosynthesis: an evolving antibiotic strategy. Molecular Biosystems, 3, 458–465.
Lloyd, A. J., Huyton, T., Turkenburg, J., & Roper, D. I. (2004). Refinement of Haemophilus influenzae diaminopimelic acid epimerase (DapF) at 1.75 Å resolution suggests a mechanism for stereocontrol during catalysis. Acta Crystallographica. Section D, Biological Crystallography, 60, 397–400.
Sagong, H. Y., & Kim, K. J. (2017). Structural basis for redox sensitivity in Corynebacterium glutamicum diaminopimelate epimerase: an enzyme involved in llysine biosynthesis. Scientific Reports, 7, 42318.
Usha, V., Dover, L. G., Roper, D. I., Fütterer, K., & Besra. (2009). Structure of the diaminopimelate epimerase DapF from Mycobacterium tuberculosis. Acta Crystallographica. Section D, Biological Crystallography, 65, 383–387.
Pillai, B., Moorthie, V. A., van Belkum, M. J., et al. (2009). Crystal structure of Diaminopimelate Epimerase from Arabidopsis thaliana, an amino acid racemase critical for l-lysine biosynthesis. Journal of Molecular Biology, 385, 580–594.
Cirilli, M., Zheng, R., Scapin, G., & Blanchard, J. S. (1998). Structural symmetry: the three-dimensional structure of Haemophilus influenzae diaminopimelate epimerase. Biochemistry, 37, 16452–16458.
Pillai, B., Cherney, M. M., Diaper, C. M. (2006). Structural insights into stereochemical inversion by diaminopimelate epimerase: an antibacterial drug target. Proceedings of the National Academy of Sciences of the United States of America, 103, 8668–8673.
Gasteiger, E., Hoogland, C., Gattiker, A., Wilkins, M. R., Appel, R. D., & Bairoch, A. (2005). In J. M. Walker (Ed.), The Proteomics Protocols Handbook, Identification and analysis tools on the ExPASy server (pp. 571–607). Humana Press.
Källberg, M., Wang, H., Wang, S., Peng, J., Wang, Z., Lu, H., et al. (2012). Template-based protein structure modeling using the RaptorX web server. Nature Protocols, 7, 1511–1522.
Hall, T. A. (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symposium Series, 41, 95–98.
Kelley, L. A., Mezulis, S., Yates, C. M., Wass, M. N., & Sternberg, M. J. (2015). The Phyre2 web portal for protein modeling, prediction and analysis. Nature Protocols, 10, 845–858.
Schrödinger, L., & DeLano, W. (2020). PyMOL. Retrieved from http://www.pymol.org/pymol
Laskowski, R. A. (2009). PDBsum new things. Nucleic Acids Research, 37, D355–D359. Retrieved from: https://doi.org/10.1093/nar/gkn860
Colovos, C., & Yeates, T. O. (1993). Verification of protein structures: patterns of nonbonded atomic interactions. Protein Science, 2, 1511–1519.
Eisenberg, D., Lüthy, R., & Bowie, J. U. (1997). VERIFY3D: Assessment of protein models with three-dimensional profiles. Methods in Enzymology, 277, 396–404.
Wiederstein, M., & Sippl, M. J. (2007). ProSA-web: interactive web service for the recognition of errors in three- dimensional structures of proteins. Nucleic Acids Research, 35, W407–W410.
Szklarczyk, D., Franceschini, A., Wyder, S., Forslund, K., Heller, D., Huerta-Cepas, J., Simonovic, M., Roth, A., Santos, A., Tsafou, K. P., & Kuhn, M. (2015). STRING v10: protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Research, 43, D447–D452.
Trott, O., & Olson, A. J. (2010). AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. Journal of Computational Chemistry, 31, 455–461.
Drews, O., Reil, G., Parlar, H., & Gorg, A. (2004). Setting up standards and a reference map for the alkaline proteome of the gram-positive bacterium Lactococcus lactis Proteomics, 4, 1293–1304.
Singh, H., Das, S., Gupta, P. P., Batra, S., Prakash, R., Srivastava, V. K., Jyoti, A., Gupta, V., Kothari, S. L., & Kaushik, S. (2020). Binding of metronidazole to Enterococcus faecalis homoserine kinase: binding studies, docking studies, and molecular dynamics simulation studies. Pharmacognosy Magazine, 16(5), 553.
Gerhart, F., Higgins, W., Tardif, C., & Ducep, J. B. (1990). 2-(4-Amino-4-carboxybutyl) aziridine-2-carboxylic acid-A potent irreversible inhibitor of diaminopimelic acid epimerase. Spontaneous formation from α-(Halomethy 1) diaminopimelic acids. Journal of Medicinal Chemistry, 33, 2157–2162.
Kushwaha, S. K., & Shakya, M. (2010). Protein interaction network analysis—approach for potential drug target identification in Mycobacterium tuberculosis. Journal of Theoretical Biology, 262, 284–294.
Caspi, R., Altman, T., Billington, R., Dreher, K., Foerster, H., Fulcher, C. A., Holland, T. A., Keseler, I. M., Kothari, A., Kubo, A., & Krummenacker, M. (2014). The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of Pathway/Genome databases. Nucleic Acids Research, 42, D459–D471.
Usha, V., Lloyd, A. J., Lovering, A. L., & Besra, G. S. (2012). Structure and function of Mycobacterium tuberculosis meso-diaminopimelic acid (DAP) biosynthetic enzymes. FEMS Microbiology Letters, 330, 10–16.
Baumann, R. J., Bohme, E. H., Wiseman, J. S., Vaal, M., & Nichols, J. S. (1988). Inhibition of Escherichia coli growth and diaminopimelic acid epimerase by 3-chlorodiaminopimelic acid. Antimicrobial Agents and Chemotherapy, 32, 1119–1123.
Vijayashree, P. J. (2019). In silico validation of the non-antibiotic drugs acetaminophen and ibuprofen as antibacterial agents against red complex pathogens. Journal of Periodontology, 90, 1441–1448.
Zimmermann, P., & Curtis, N. (2017). Antimicrobial effects of antipyretics. Antimicrobial Agents and Chemotherapy, 61, 02268–02216.
Abdul-Hussein, Z. R. (2014). Acetaminophen as antibacterial agent and its effect on antibiotic susceptibilities of some pathogenic bacteria. European Journal of Experimental Biology, 4, 351–356.
Neher, A., Arnitz, R., Gstöttner, M., Schä, D., Kröss, E. M., & Nagl, M. (2008). Antimicrobial activity of dexamethasone and its combination with N-chlorotaurine. Archives of Otolaryngology - Head and Neck Surgery, 134, 615–620.
Skariyachan, S., Manjunath, M., & Bachappanavar, N. (2019). Screening of potential lead molecules against prioritised targets of multi-drug-resistant-Acinetobacter baumannii–insights from molecular docking, molecular dynamic simulations and in vitro assays. Journal of Biomolecular Structure & Dynamics, 37, 1146–1169.
Singh, H., Das, S., Yadav, J., Srivastava, V. K., Jyoti, A., & Kaushik, S. (2021). In silico prediction, molecular docking and binding studies of acetaminophen and dexamethasone to Enterococcus faecalis diaminopimelate epimerase. Journal of Molecular Recognition, 34, e2894.
Fuchs, T. M., Schneider, B., Krumbach, K., Eggeling, L., & Gross, R. (2000). Characterization of a Bordetella pertussis diaminopimelate (DAP) biosynthesis locus identifies dapC, a novel gene coding for an N-succinyl-L, L-DAP aminotransferase. Journal of Bacteriology, 182, 3626–3631.
Acknowledgements
This work was supported by the Department of Biotechnology and Microbiology, School of Sciences, Noida International University, Gautam Budh Nagar, U.P., India.
Author information
Authors and Affiliations
Contributions
Shilpy Singh - Conception or design of the work, Data analysis, interpretation, prepared figures and wrote and reviewed the main manuscript text.
Afsana Praveen - Conception or design of the work, wrote and reviewed the main manuscript text.
Suruchi M. Khanna - Data analysis, interpretation, prepared figures and reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singh, S., Praveen, A. & Khanna, S.M. Computational Modelling, Functional Characterization and Molecular Docking to Lead Compounds of Bordetella pertussis Diaminopimelate Epimerase. Appl Biochem Biotechnol 195, 6675–6693 (2023). https://doi.org/10.1007/s12010-023-04413-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04413-0