Abstract
Orthopedic infections due to biofilm formation in biomaterial-based implants have become challenging in bone tissue engineering. In the present study, in vitro antibacterial analysis of amino-functionalized MCM-48 mesoporous silica nanoparticles (AF-MSNs) loaded with vancomycin is analyzed for its potential as a drug carrier for the sustained/controlled release of vancomycin against Staphylococcus aureus. The effective incorporation of vancomycin into the inner core of AF-MSNs was observed by alternation in the absorption frequencies obtained by Fourier transform infrared spectroscopy (FTIR). Dynamic light scattering (DLS) and high resolution-transmission electron microscopy (HR-TEM) results show that all the AF-MSNs had homogeneous spherical shapes with a mean diameter of 165.2 ± 1.25 nm, and there is a slight change in the hydrodynamic diameter after vancomycin loading. Furthermore, the zeta potential of all the AF-MSNs (+ 30.5 ± 0.54 mV) and AF-MSN/VA (+ 33.3 ± 0.56 mV) were positively charged due to effective functionalization with 3-aminopropyl triethoxysilane (APTES). Furthermore, cytotoxicity results show that the AF-MSNs have better biocompatibility than non-functionalized MSNs (p < 0.05), and results prove AF-MSNs loaded with vancomycin show better antibacterial effect against S. aureus than non-functionalized MSNs. Results confirm that bacterial membrane integrity was affected by treatment with AF-MSNs and AF-MSN/VA by staining the treated cells with FDA/PI. Field emission scanning electron microscopy (FESEM) analysis confirmed the shrinkage of bacterial cells and membrane disintegration. Furthermore, these results demonstrate that amino-functionalized MSNs loaded with vancomycin significantly increased the anti-biofilm and biofilm inhibitory effect and can be incorporated with biomaterial-based bone substitutes and bone cement to prevent orthopedic infections post-implantation.
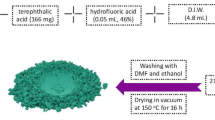
Graphical Abstract















Similar content being viewed by others
Data Availability
The authors confirm that the data analyzed and generated from these research findings are provided within this article.
References
Batailler, C., Swan, J., SappeyMarinier, E., Servien, E., & Lustig, S. (2021). New technologies in knee arthroplasty: Current concepts. Journal of Clinical Medicine, 10(1), 1–18. https://doi.org/10.3390/jcm10010047
Fernandez de Grado, G., Keller, L., Idoux-Gillet, Y., Wagner, Q., Musset, A. M., Benkirane-Jessel, N., … Offner, D. (2018). Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. Journal of Tissue Engineering, 9. https://doi.org/10.1177/2041731418776819
Geurts, J., Chris Arts, J. J., & Walenkamp, G. H. I. M. (2011). Bone graft substitutes in active or suspected infection Contra-indicated or not? Injury, 42(SUPPL 2), S82–S86. https://doi.org/10.1016/j.injury.2011.06.189
Otto-Lambertz, C., Yagdiran, A., Wallscheid, F., Eysel, P., & Jung, N. (2017). Periprosthetic infection in joint replacement - Diagnosis and treatment. Deutsches Arzteblatt International, 114(20), 347–354. https://doi.org/10.3238/arztebl.2017.0347
Ribeiro, M., Monteiro, F. J., & Ferraz, M. P. (2012). Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. Biomatter, 2(4), 176–194. https://doi.org/10.4161/biom.22905
Allizond, V., Comini, S., Cuffini, A. M., & Banche, G. (2022). Current knowledge on biomaterials for orthopedic applications modified to reduce bacterial adhesive ability. Antibiotics, 11(4). https://doi.org/10.3390/antibiotics11040529
Krismer, M. (2012). Definition of infection. HIP. International, 22(SUPPL 8), 2–5. https://doi.org/10.5301/HIP.2012.9563
Brescó, M. S., Harris, L. G., Thompson, K., Stanic, B., Morgenstern, M., O’Mahony, L., … Moriarty, T. F. (2017). Pathogenic mechanisms and host interactions in Staphylococcus epidermidis device-related infection. Frontiers in Microbiology, 8(AUG). https://doi.org/10.3389/fmicb.2017.01401
Glage, S., Paret, S., Winkel, A., Stiesch, M., Bleich, A., Krauss, J. K., & Schwabe, K. (2017). A new model for biofilm formation and inflammatory tissue reaction: Intraoperative infection of a cranial implant with Staphylococcus aureus in rats. Acta Neurochirurgica, 159(9), 1747–1756. https://doi.org/10.1007/s00701-017-3244-7
Seebach, E., & Kubatzky, K. F. (2019). Chronic implant-related bone infections-Can Immune modulation be a therapeutic strategy? Frontiers in immunology, 10(July), 1724. https://doi.org/10.3389/fimmu.2019.01724
Arduino, J. M., Kaye, K. S., Reed, S. D., Peter, S. A., Sexton, D. J., Chen, L. F., … Anderson, D. J. (2015). Staphylococcus aureus infections following knee and hip prosthesis insertion procedures. Antimicrobial Resistance and Infection Control, 4(1), 1–7. https://doi.org/10.1186/s13756-015-0057-4
Hudson, M. C., Ramp, W. K., & Frankenburg, K. P. (1999). Staphylococcus aureus adhesion to bone matrix and bone-associated biomaterials. FEMS Microbiology Letters, 173(2), 279–284. https://doi.org/10.1016/S0378-1097(99)00079-8
Uribe-García, A., Paniagua-Contreras, G. L., Monroy-Pérez, E., Bustos-Martínez, J., Hamdan-Partida, A., Garzón, J., … Vaca, S. (2021). Frequency and expression of genes involved in adhesion and biofilm formation in Staphylococcus aureus strains isolated from periodontal lesions. Journal of Microbiology, Immunology and Infection, 54(2), 267–275. https://doi.org/10.1016/j.jmii.2019.05.010
Slane, J., Vivanco, J., Rose, W., Ploeg, H. L., & Squire, M. (2015). Mechanical, material, and antimicrobial properties of acrylic bone cement impregnated with silver nanoparticles. Materials Science and Engineering C, 48, 188–196. https://doi.org/10.1016/j.msec.2014.11.068
Sawant, S. N., Selvaraj, V., Prabhawathi, V., & Doble, M. (2013). Antibiofilm properties of silver and gold incorporated PU, PCLm, PC and PMMA nanocomposites under two shear conditions. PLoS ONE, 8(5), 1–9. https://doi.org/10.1371/journal.pone.0063311
Wekwejt, M., Michno, A., Truchan, K., Pałubicka, A., Świeczko-żurek, B., Osyczka, A. M., & Zieliński, A. (2019). Antibacterial activity and cytocompatibility of bone cement enriched with antibiotic, nanosilver, and nanocopper for bone regeneration. Nanomaterials, 9(8). https://doi.org/10.3390/nano9081114
Kargozar, S., Montazerian, M., Hamzehlou, S., Kim, H. W., & Baino, F. (2018). Mesoporous bioactive glasses: Promising platforms for antibacterial strategies. Acta Biomaterialia (Vol. 81). https://doi.org/10.1016/j.actbio.2018.09.052
Kühn, K. D., Renz, N., & Trampuz, A. (2017). Lokale Antibiotikatherapie. Der Unfallchirurg, 120(7), 561–572. https://doi.org/10.1007/s00113-017-0372-8
Liu, Y., Zeng, Y., Wu, Y., Li, M., Xie, H., & Shen, B. (2021). A comprehensive comparison between cementless and cemented fixation in the total knee arthroplasty: An updated systematic review and meta-analysis. Journal of Orthopaedic Surgery and Research, 16(1), 1–14. https://doi.org/10.1186/s13018-021-02299-4
Bistolfi, A., Massazza, G., Verné, E., Massè, A., Deledda, D., Ferraris, S., … Crova, M. (2011). Antibiotic-loaded cement in orthopedic surgery: A review. ISRN Orthopedics, 2011, 1–8. https://doi.org/10.5402/2011/290851
Coraça-Huber, D. C., Steixner, S. J. M., Najman, S., Stojanovic, S., Finze, R., Rimashevskiy, D., … Schnettler, R. (2022). Lyophilized human bone allograft as an antibiotic carrier: An in vitro and in vivo study. Antibiotics, 11(7), 1–16. https://doi.org/10.3390/antibiotics11070969
Gisbert-Garzarán, M., Manzano, M., & Vallet-Regí, M. (2020). Mesoporous silica nanoparticles for the treatment of complex bone diseases: Bone cancer, bone infection and osteoporosis. Pharmaceutics, 12(1). https://doi.org/10.3390/pharmaceutics12010083
Bernardos, A., Piacenza, E., Sancenón, F., Hamidi, M., Maleki, A., Turner, R. J., & Martínez-Máñez, R. (2019). Mesoporous silica-based materials with bactericidal properties. Small (Weinheim an der Bergstrasse, Germany), 15(24), 1–34. https://doi.org/10.1002/smll.201900669
Mousavi Elyerdi, S. M., Sarvi, M. N., & O’Connor, A. J. (2019). Synthesis of ultra small nanoparticles (< 50 nm) of mesoporous MCM-48 for bio-adsorption. Journal of Porous Materials, 26(3), 839–846. https://doi.org/10.1007/s10934-018-0650-z
Ezzati, N., Mahjoub, A. R., AbolhosseiniShahrnoy, A., & Syrgiannis, Z. (2019). Amino Acid-functionalized hollow mesoporous silica nanospheres as efficient biocompatible drug carriers for anticancer applications. International Journal of Pharmaceutics, 572, 118709. https://doi.org/10.1016/j.ijpharm.2019.118709
Gan, Q., Dai, D., Yuan, Y., Qian, J., Sha, S., Shi, J., & Liu, C. (2012). Effect of size on the cellular endocytosis and controlled release of mesoporous silica nanoparticles for intracellular delivery. Biomedical Microdevices, 14(2), 259–270. https://doi.org/10.1007/s10544-011-9604-9
Coelho, P., Oliveira, J., Fernandes, I., Araújo, P., Pereira, A. R., Gameiro, P., & Bessa, L. J. (2021). Pyranoanthocyanins interfering with the quorum sensing of pseudomonas aeruginosa and staphylococcus aureus. International Journal of Molecular Sciences, 22(16). https://doi.org/10.3390/ijms22168559
Birtekocak, F., Demirbolat, G. M., & Cevik, O. (2021). TRAIL conjugated silver nanoparticle synthesis, characterization and therapeutic effects on HT-29 colon cancer cells. Iranian Journal of Pharmaceutical Research, 20(2), 45–56. https://doi.org/10.22037/ijpr.2020.112069.13514
Nikolić, B., Vasilijević, B., Mitić-Culafić, D., Lesjak, M., Vuković-Gačić, B., Dukić, N. M., & Knežević-Vukčević, J. (2016). Screening of the antibacterial effect of Juniperus sibirica and Juniperus sabina essential oils in a microtitre platebased MIC assay. Botanica Serbica. https://doi.org/10.5281/zenodo.48858
Nie, B., Huo, S., Qu, X., Guo, J., Liu, X., Hong, Q., … Yue, B. (2022). Bone infection site targeting nanoparticle-antibiotics delivery vehicle to enhance treatment efficacy of orthopedic implant related infection. Bioactive Materials, 16(December 2021), 134–148. https://doi.org/10.1016/j.bioactmat.2022.02.003
Burygin, G. L., Khlebtsov, B. N., Shantrokha, A. N., Dykman, L. A., Bogatyrev, V. A., & Khlebtsov, N. G. (2009). On the enhanced antibacterial activity of antibiotics mixed with gold nanoparticles. Nanoscale Research Letters, 4(8), 794–801. https://doi.org/10.1007/s11671-009-9316-8
Kumari, M., Shukla, S., Pandey, S., Giri, V. P., Bhatia, A., Tripathi, T., … Mishra, A. (2017). Enhanced cellular internalization: A bactericidal mechanism more relative to biogenic nanoparticles than chemical counterparts. ACS Applied Materials and Interfaces, 9(5), 4519–4533. https://doi.org/10.1021/acsami.6b15473
Sana, S., Datta, S., Biswas, D., & Sengupta, D. (2018). Assessment of synergistic antibacterial activity of combined biosurfactants revealed by bacterial cell envelop damage. Biochimica et Biophysica Acta - Biomembranes, 1860(2), 579–585. https://doi.org/10.1016/j.bbamem.2017.09.027
Mu, H., Tang, J., Liu, Q., Sun, C., Wang, T., & Duan, J. (2016). Potent antibacterial nanoparticles against biofilm and intracellular bacteria. Scientific Reports, 6(2015). https://doi.org/10.1038/srep18877
Kim, T. W., Chung, P. W., & Lin, V. S. Y. (2010). Facile synthesis of monodisperse spherical MCM-48 mesoporous silica nanoparticles with controlled particle size. Chemistry of Materials, 22(17), 5093–5104. https://doi.org/10.1021/cm1017344
Estevão, B. M., Miletto, I., Hioka, N., Marchese, L., & Gianotti, E. (2021). Mesoporous silica nanoparticles functionalized with amino groups for biomedical applications. ChemistryOpen, 10(12), 1251–1259. https://doi.org/10.1002/open.202100227
Zatorska, M., Łazarski, G., Maziarz, U., Wilkosz, N., Honda, T., Yusa, S. ichi, … Kepczynski, M. (2020). Drug-loading capacity of polylactide-based micro- and nanoparticles – Experimental and molecular modeling study. International Journal of Pharmaceutics, 591(October). https://doi.org/10.1016/j.ijpharm.2020.120031
Mai, Z., Chen, J., Hu, Y., Liu, F., Fu, B., Zhang, H., … Zhou, W. (2017). Novel functional mesoporous silica nanoparticles loaded with Vitamin E acetate as smart platforms for pH responsive delivery with high bioactivity. Journal of Colloid and Interface Science, 508, 184–195. https://doi.org/10.1016/j.jcis.2017.07.027
ZakeriSiavashani, A., HaghbinNazarpak, M., Fayyazbakhsh, F., Toliyat, T., McInnes, S. J. P., & Solati-Hashjin, M. (2016). Effect of amino-functionalization on insulin delivery and cell viability for two types of silica mesoporous structures. Journal of Materials Science, 51(24), 10897–10909. https://doi.org/10.1007/s10853-016-0301-1
He, Y., Luo, L., Liang, S., Long, M., & Xu, H. (2017). Amino-functionalized mesoporous silica nanoparticles as efficient carriers for anticancer drug delivery. Journal of Biomaterials Applications, 32(4), 524–532. https://doi.org/10.1177/0885328217724638
Muriel-Galet, V., Pérez-Esteve, É., Ruiz-Rico, M., Martínez-Máñez, R., Barat, J. M., Hernández-Muñoz, P., & Gavara, R. (2018). Anchoring gated mesoporous silica particles to ethylene vinyl alcohol films for smart packaging applications. Nanomaterials, 8(10). https://doi.org/10.3390/nano8100865
Majoul, N., Aouida, S., & Bessaïs, B. (2015). Progress of porous silicon APTES-functionalization by FTIR investigations. Applied Surface Science, 331, 388–391. https://doi.org/10.1016/j.apsusc.2015.01.107
Juère, E., Caillard, R., & Kleitz, F. (2020). Pore confinement and surface charge effects in protein-mesoporous silica nanoparticles formulation for oral drug delivery. Microporous and Mesoporous Materials, 306. https://doi.org/10.1016/j.micromeso.2020.110482
Zaharudin, N. S., Mohamed Isa, E. D., Ahmad, H., Abdul Rahman, M. B., & Jumbri, K. (2020). Functionalized mesoporous silica nanoparticles templated by pyridinium ionic liquid for hydrophilic and hydrophobic drug release application. Journal of Saudi Chemical Society, 24(3), 289–302. https://doi.org/10.1016/j.jscs.2020.01.003
Liu, J. X., Bravo, D., Buza, J., Kirsch, T., Kennedy, O., Rokito, A., … Virk, M. S. (2018). Topical vancomycin and its effect on survival and migration of osteoblasts, fibroblasts, and myoblasts: An in vitro study. Journal of Orthopaedics, 15(1), 53–58. https://doi.org/10.1016/j.jor.2018.01.032
Kaur, A., Preet, S., Kumar, V., Kumar, R., & Kumar, R. (2019). Synergetic effect of vancomycin loaded silver nanoparticles for enhanced antibacterial activity. Colloids and Surfaces B: Biointerfaces, 176, 62–69. https://doi.org/10.1016/j.colsurfb.2018.12.043
Tawakoli, P. N., Al-Ahmad, A., Hoth-Hannig, W., Hannig, M., & Hannig, C. (2013). Comparison of different live/dead stainings for detection and quantification of adherent microorganisms in the initial oral biofilm. Clinical Oral Investigations, 17(3), 841–850. https://doi.org/10.1007/s00784-012-0792-3
Quinn, C. F., & Hansen, L. D. (2019). Microcalorimetry of biological molecules (Vol. 1964). Retrieved from http://link.springer.com/https://doi.org/10.1007/978-1-4939-9179-2
Vejzovic, D., Piller, P., Cordfunke, R. A., Drijfhout, J. W., Eisenberg, T., Lohner, K., & Malanovic, N. (2022). Where electrostatics matter: Bacterial surface neutralization and membrane disruption by antimicrobial peptides SAAP-148 and OP-145. Biomolecules, 12(9). https://doi.org/10.3390/biom12091252
Michailidis, M., Sorzabal-Bellido, I., Adamidou, E. A., Diaz-Fernandez, Y. A., Aveyard, J., Wengier, R., … Shchukin, D. (2017). Modified mesoporous silica nanoparticles with a dual synergetic antibacterial effect. ACS Applied Materials and Interfaces, 9(44), 38364–38372. https://doi.org/10.1021/acsami.7b14642
Liu, Y., Qin, R., Zaat, S. A. J., Breukink, E., & Heger, M. (2015). Antibacterial photodynamic therapy: Overview of a promising approach to fight antibiotic-resistant bacterial infections. Journal of Clinical and Translational Research, 1(3), 140–167. https://doi.org/10.18053/jctres.201503.002
Yang, C., Xie, H., Li, Q. C., Sun, E. J., & Su, B. L. (2015). Adherence and interaction of cationic quantum dots on bacterial surfaces. Journal of Colloid and Interface Science, 450, 388–395. https://doi.org/10.1016/j.jcis.2015.03.041
Acknowledgements
The authors thank the Director, CSIR-CLRI, for his support in conducting this research, carrying out experimental work, and publishing this article (CSIR-CLRI communication No. 1774). The authors also acknowledge and thank the support and help rendered by CLRI-CATERS (Centre for Analysis, Testing, Evaluation and Reporting Services) for this research work.
Funding
This work was supported by the Council of Scientific and Industrial Research (CSIR), New Delhi, India, by providing fellowship through the award of Senior Research Fellowship (File No. 31/006(0467)/2019-EMR-I) to Mr. Syed Nasar Rahaman, and the authors are grateful to CSIR-CLRI for funding this research through the Major Laboratory Project (MLP-03), and CSIR-Focused Basic Research (FBR) MLP-2006.
Author information
Authors and Affiliations
Contributions
SNR: contributed to conceptualization, hypothesis, study design, experimentation, material characterization, data interpretation, analysis of the data, and manuscript preparation.
SP: data interpretation and experimental analysis.
AS: performed the material preparation and assisted in vitro studies.
SKAS: contributed to the study design, data interpretation, and final manuscript review.
All authors approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rahaman, S.N., Pathmanapan, S., Sidharthan, A. et al. Vancomycin Loaded Amino-Functionalized MCM-48 Mesoporous Silica Nanoparticles as a Promising Drug Carrier in Bone Substitutes for Bacterial Infection Management. Appl Biochem Biotechnol 195, 6607–6632 (2023). https://doi.org/10.1007/s12010-023-04406-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04406-z