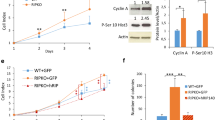
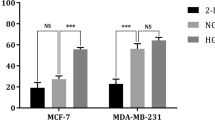
Abstract
Breast cancer is the frequently diagnosed cancer and the leading cancer death among women. The growing tumour of the breast is composed of both normoxic and hypoxic cells, and the heterogeneity of tumour affects the targeted treatment strategies against breast cancer. The functional and therapeutic status of the Warburg effect is mostly recognized, and the genes involved in glycolysis have become a target for anticancer therapeutic strategies. Glut-1 is essential for basal glucose uptake among the glucose transporters and could act as a potential target for anticancer therapy. In the present study, we explored the alteration in the metabolic phenotype of SKBR-3 cells, representing HER-2 overexpressed breast cancer cell line, with Glut-1 inhibition by a synthetic small molecule inhibitor WZB117 in the presence or absence of cobalt chloride (CoCl2) induced biochemical hypoxia in vitro. We found that WZB117 and CoCl2 in combination could inhibit metabolic phenotype characteristics such as glucose uptake, cell migration, lactate and ATP production in SKBR-3 cells. Also, Glut-1 inhibition induced apoptosis and cell cycle arrest at the G0–G1 phase even under CoCl2-induced mimic hypoxia. Our findings suggest that Glut-1 inhibition by WZB117 could overcome the protective effect of CoCl2 mimic hypoxia by regulating glycolysis and altering the metabolic phenotype of breast cancer cells. The considering excellent efficacy and minimal toxicity suggest that WZB117 may be a promising anticancer drug to the current therapies.







Similar content being viewed by others
Data Availability
Data will be available on request.
References
Siegel, R. L., Miller, K. D., & Jemal, A. (2019). Cancer statistics, 2019. CA: A Cancer Journal for Clinicians, 69, 7–34.
Tan, J., & Le, A. (2021). in The Heterogeneity of Cancer Metabolism (pp. 89–101). Springer.
Park, S., Koo, J. S., Kim, M. S., Park, H. S., Lee, J. S., Lee, J. S., Kim, S. I., & Park, B.-W. (2012). Characteristics and outcomes according to molecular subtypes of breast cancer as classified by a panel of four biomarkers using immunohistochemistry. The Breast, 21, 50–57.
Lenz, G., Hamilton, A., Geng, S., Hong, T., Kalkum, M., Momand, J., Kane, S. E., & Huss, J. M. (2018). t-Darpp Activates IGF-1R Signaling to Regulate Glucose Metabolism in Trastuzumab-Resistant Breast Cancer Cellst-Darpp, IGF-1R Signaling, and Glucose Metabolism. Clinical Cancer Research, 24, 1216–1226.
Engel, R. H., & Kaklamani, V. G. (2007). HER2-positive breast cancer. Drugs, 67, 1329–1341.
Hanahan, D., & Weinberg, R. A. (2011). Hallmarks of cancer: the next generation. Cell, 144, 646–674.
Warburg, O., Wind, F., & Negelein, E. (1927). The metabolism of tumors in the body. The Journal of general physiology, 8, 519.
Liberti, M. V., & Locasale, J. W. (2016). The Warburg effect: How does it benefit cancer cells? Trends in biochemical sciences, 41, 211–218.
Ancey, P. B., Contat, C., & Meylan, E. (2018). Glucose transporters in cancer–from tumor cells to the tumor microenvironment. The FEBS journal, 285, 2926–2943.
Joost, H.-G., Bell, G. I., Best, J. D., Birnbaum, M. J., Charron, M. J., Chen, Y., Doege, H., James, D. E., Lodish, H. F., Moley, K.H.J.A.J.O.P.-E., Metabolism. (2002). Nomenclature of the GLUT/SLC2A family of sugar/polyol transport facilitators. American Journal of Physiology-Endocrinology And Metabolism, 282, E974–E976.
Takata, K., Kasahara, T., Kasahara, M., Ezaki, O., Hirano, H. J. B., communications br. (1990). Erythrocyte/HepG2-type glucose transporter is concentrated in cells of blood-tissue barriers. Biochemical and biophysical research communications, 173, 67–73.
Carvalho, K. C., Cunha, I. W., Rocha, R. M., Ayala, F. R., Cajaíba, M. M., Begnami, M. D., Vilela, R. S., Paiva, G. R., Andrade, R. G., & Soares, F. A. (2011). GLUT1 expression in malignant tumors and its use as an immunodiagnostic marker. Clinics, 66, 965–972.
Brown, R. S., & Wahl, R. L. (1993). Overexpression of glut-1 glucose transporter in human breast cancer an immunohistochemical study. Cancer, 72, 2979–2985.
Wang, J., Ye, C., Chen, C., Xiong, H., Xie, B., Zhou, J., Chen, Y., Zheng, S., & Wang, L. (2017). Glucose transporter GLUT1 expression and clinical outcome in solid tumors: A systematic review and meta-analysis. Oncotarget, 8, 16875.
Graham, N. A., Tahmasian, M., Kohli, B., Komisopoulou, E., Zhu, M., Vivanco, I., Teitell, M. A., Wu, H., Ribas, A., & Lo, R. S. (2012). Glucose deprivation activates a metabolic and signaling amplification loop leading to cell death. Molecular systems biology, 8, 589.
Suzuki, S., Okada, M., Takeda, H., Kuramoto, K., Sanomachi, T., Togashi, K., Seino, S., Yamamoto, M., Yoshioka, T., & Kitanaka, C. (2018). Involvement of GLUT1-mediated glucose transport and metabolism in gefitinib resistance of non-small-cell lung cancer cells. Oncotarget, 9, 32667.
Liu, Y., Cao, Y., Zhang, W., Bergmeier, S., Qian, Y., Akbar, H., Colvin, R., Ding, J., Tong, L., & Wu, S. (2012). A Small-Molecule Inhibitor of Glucose Transporter 1 Downregulates Glycolysis, Induces Cell-Cycle Arrest, and Inhibits Cancer Cell Growth In Vitro and In VivoA Glut1 Inhibitor Reduces Cancer Growth In Vitro and In Vivo. Molecular cancer therapeutics, 11, 1672–1682.
Liu, W., Fang, Y., Wang, X.-T., Liu, J., Dan, X., & Sun, L.-L. (2014). Overcoming 5-Fu resistance of colon cells through inhibition of Glut1 by the specific inhibitor WZB117. Asian Pacific Journal of Cancer Prevention, 15, 7037–7041.
Zhao, F., Ming, J., Zhou, Y., Fan, L. J. C. C., pharmacology. (2016). Inhibition of Glut1 by WZB117 sensitizes radioresistant breast cancer cells to irradiation. Cancer chemotherapy and pharmacology, 77, 963–972.
Jang, S. M., Han, H., Jang, K.-S., Jun, Y. J., Jang, S.-H., Min, K.-W., Chung, M. S., & Paik, S. S. (2012). The glycolytic phenotype is correlated with aggressiveness and poor prognosis in invasive ductal carcinomas. Journal of Breast Cancer, 15, 172–180.
Hussein, Y. R., Bandyopadhyay, S., Semaan, A., Ahmed, Q., Albashiti, B., Jazaerly, T., Nahleh, Z., & Ali-Fehmi, R. (2011). Glut-1 expression correlates with basal-like breast cancer. Translational oncology, 4, 321–327.
Denko, N. C. (2008). Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nature Reviews Cancer, 8, 705–713.
Axelson, H., Fredlund, E., Ovenberger, M., Landberg, G., & Påhlman, S. (2005). Hypoxia-induced dedifferentiation of tumor cells—a mechanism behind heterogeneity and aggressiveness of solid tumors. Seminars in Cell & Developmental Biology, 16, 554–563.
Rastogi, S., Banerjee, S., Chellappan, S., & Simon, G. R. (2007). Glut-1 antibodies induce growth arrest and apoptosis in human cancer cell lines. Cancer letters, 257, 244–251.
Okcu, O., Sen, B., Ozturk, C., Guvendi, G. F., & Bedir, R. (2022) GLUT-1 expression in breast cancer. Turkish Journal of Pathology, 38 , 114–121.
Kang, S. S., Chun, Y. K., Hur, M. H., Lee, H. K., Kim, Y. J., Hong, S. R., Lee, J. H., Lee, S. G., & Park, Y. K. (2002). Clinical significance of glucose transporter 1 (GLUT1) expression in human breast carcinoma. Japanese Journal of Cancer Research, 93, 1123–1128.
Suteau, V., Bukasa-Kakamba, J., Virjogh-Cenciu, B., Adenis, A., Sabbah, N., & Drak Alsibai, K. (2022). Pathological Significance of GLUT-1 Expression in Breast Cancer Cells in Diabetic and Obese Patients: The French Guiana Study. Cancers, 14, 437.
Zhou, S. H., Fan, J., Chen, X. M., Cheng, K. J., Wang, S. Q. J. H., Sciences, N. J., & f. t., Head, S. o. t. and Neck. (2009). Inhibition of cell proliferation and glucose uptake in human laryngeal carcinoma cells by antisense oligonucleotides against glucose transporter-1. Head & Neck: Journal for the Sciences and Specialties of the Head and Neck, 31, 1624–1633.
Ojelabi, O. A., Lloyd, K. P., Simon, A. H., De Zutter, J. K., & Carruthers, A. (2016). WZB117 (2-Fluoro-6-(m-hydroxybenzoyloxy) Phenyl m-Hydroxybenzoate) inhibits GLUT1-mediated sugar transport by binding reversibly at the exofacial sugar binding site. Journal of Biological Chemistry, 291, 26762–26772.
Ždralević, M., Marchiq, I., de Padua, M. M. C., Parks, S. K., & Pouysségur, J. (2017). Metabolic plasiticy in cancers—distinct role of glycolytic enzymes GPI, LDHs or membrane transporters MCTs. Frontiers in oncology, 7, 313.
Farhadi, P., Yarani, R., Valipour, E., Kiani, S., Hoseinkhani, Z., Mansouri, K. J. B., Pharmacotherapy. (2022). Cell line-directed breast cancer research based on glucose metabolism status. Biomedicine & Pharmacotherapy, 146, 112526.
Peng, Y., Xing, S.-N., Tang, H.-Y., Wang, C.-D., Yi, F.-P., Liu, G.-L., & Wu, X.-M. (2019). Influence of glucose transporter 1 activity inhibition on neuroblastoma in vitro. Gene, 689, 11–17.
Muñoz-Sánchez, J., & Chánez-Cárdenas, M. E. (2019). The use of cobalt chloride as a chemical hypoxia model. Journal of Applied Toxicology, 39, 556–570.
Pérez-Tomás, R., & Pérez-Guillén, I. (2020). Lactate in the tumor microenvironment: An essential molecule in cancer progression and treatment. Cancers, 12, 3244.
Goetze, K., Walenta, S., Ksiazkiewicz, M., Kunz-Schughart, L. A., & Mueller-Klieser, W. (2011). Lactate enhances motility of tumor cells and inhibits monocyte migration and cytokine release. International journal of oncology, 39, 453–463.
Ganapathy-Kanniappan, S., & Geschwind, J.-F.H. (2013). Tumor glycolysis as a target for cancer therapy: Progress and prospects. Molecular cancer, 12, 1–11.
Jose, C., Bellance, N., & Rossignol, R.J.B.E.B.A.-B. (2011). Choosing between glycolysis and oxidative phosphorylation: a tumor’s dilemma? Biochimica et Biophysica Acta (BBA) -Bioenergetics, 1807, 552–561.
San-Millán, I., Julian, C. G., Matarazzo, C., Martinez, J., & Brooks, G. A. (2020). Is lactate an oncometabolite? Evidence supporting a role for lactate in the regulation of transcriptional activity of cancer-related genes in MCF7 breast cancer cells. Frontiers in Oncology, 9, 1536.
Ren, Y., & Shen, H.-M. (2019). Critical role of AMPK in redox regulation under glucose starvation. Redox Biology, 25, 101154.
Zhao, Y., Butler, E. B., Tan, M. J. C. D., disease. (2013). Targeting cellular metabolism to improve cancer therapeutics. Cell death & disease, 4, e532–e532.
Andrisse, S., Koehler, R. M., Chen, J. E., Patel, G. D., Vallurupalli, V. R., Ratliff, B. A., Warren, D. E., & Fisher, J. S. (2014). Role of GLUT1 in regulation of reactive oxygen species. Redox biology, 2, 764–771.
Kim, J.-W., Tchernyshyov, I., Semenza, G. L., & Dang, C. V. (2006). HIF-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell metabolism, 3, 177–185.
Liu, H., Savaraj, N., Priebe, W., & Lampidis, T. J. (2002). Hypoxia increases tumor cell sensitivity to glycolytic inhibitors: A strategy for solid tumor therapy (Model C). Biochemical pharmacology, 64, 1745–1751.
Li, Y.-L., Weng, H.-C., Hsu, J.-L., Lin, S.-W., Guh, J.-H., & Hsu, L.-C. (2019). The combination of MK-2206 and WZB117 exerts a synergistic cytotoxic effect against breast cancer cells. Frontiers in Pharmacology, 10, 1311.
Wei, M., Lu, L., Sui, W., Liu, Y., Shi, X., Lv, L. J. B., communications br. (2018). Inhibition of GLUTs by WZB117 mediates apoptosis in blood-stage Plasmodium parasites by breaking redox balance. Biochemical and biophysical research communications, 503, 1154–1159.
Funding
Author A.B.L. has received research support from University Grants Commission, Government of India (F.16–6(DEC.2016)/2017(NET)); UGC-Ref. No. 951/(OBC)(CSIR-UGC NET DEC.2016).
Author information
Authors and Affiliations
Contributions
A.B.L and L.S. contributed to the study conception and design. Experimentation, data collection, and analysis were performed by A.B.L. Flow cytometric analysis and microscopic imaging were assisted by S.T.P. and G.R.A. The first draft of the manuscript was written by A.B.L. Manuscript was revised and edited by L.S. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Littleflower, A.B., Antony, G.R., Parambil, S.T. et al. Metabolic Phenotype Intricacies on Altered Glucose Metabolism of Breast Cancer Cells upon Glut-1 Inhibition and Mimic Hypoxia In Vitro. Appl Biochem Biotechnol 195, 5838–5854 (2023). https://doi.org/10.1007/s12010-023-04373-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04373-5