Abstract
The limitations of graft material, and surgical sites for autografts in bone defects treatment have become a significant challenge in bone tissue engineering. Phytocompounds markedly affect bone metabolism by activating the osteogenic signaling pathways. The present study investigated the biocompatibility of the bio-composite thermo-responsive hydrogels consisting of chitosan (CS), and methylcellulose (MC) encapsulated with veratric acid (VA) as a restorative agent for bone defect treatment. The spectroscopy analyses confirmed the formation of CS/MC hydrogels and VA encapsulated CS/MC hydrogels (CS/MC-VA). Molecular analysis of the CS-specific MC decamer unit with VA complex exhibited a stable integration in the system. Further, Runx2 (runt-related transcription factor 2) was found in the docking mechanism with VA, indicating a high binding affinity towards the functional site of the Runx2 protein. The formulated CS/MC-VA hydrogels exhibited biocompatibility with the mouse mesenchymal stem cells, while VA promoted osteogenic differentiation in the stem cells, which was verified by calcium phosphate deposition through the von Kossa staining. The study results suggest that CS/MC-VA could be a potential therapeutic alternative source for bone regeneration.
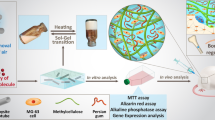
Graphical Abstract









Similar content being viewed by others
Data Availability
Data that support the findings of this study will be available from the corresponding author upon reasonable request.
References
Crockett, J. C., Rogers, M. J., Coxon, F. P., Hocking, L. J., & Helfrich, M. H. (2011). Bone remodelling at a glance. Journal of Cell Science, 124(7), 991–998.
Florencio-Silva, R., da Sasso, G. R. S., Sasso-Cerri, E., Simões, M. J., & Cerri, P. S. (2015). Biology of bone tissue: Structure, Function, and factors that influence bone cells. BioMed Research International, 421746, 1–17.
Holmes, D. (2017). Non-union bone fracture: A quicker fix. Nature, 550, S193–S193.
Hunter, D. J., McDougall, J. J., & Keefe, F. J. (2008). The symptoms of osteoarthritis and the genesis of pain. Rheumatic Diseases Clinics of North America, 34(3), 623–643.
Lu, T., Li, Y., & Chen, T. (2013). Techniques for fabrication and construction of three-dimensional scaffolds for tissue engineering. International Journal of Nanomedicine, 8(1), 337–350.
Rizwan, M., Yahya, R., Hassan, A., Yar, M., Omar, A. R., Azari, P., Azzahari, A. D., Selvanathan, V., Al-Maleki, A. R., & Venkatraman, G. (2018). Synthesis of a novel organosoluble, biocompatible, and antibacterial chitosan derivative for biomedical applications. Journal of Applied Polymer Science, 135(9), 45905.
Manjunath, K. S., Sridhar, K., Gopinath, V., Sankar, K., Sundaram, A., Gupta, N., Shiek, A. S. S. J., & Shantanu, P. S. (2020). Facile manufacturing of fused-deposition modeled composite scaffolds for tissue engineering-an embedding model with plasticity for incorporation of additives. Biomedical Materials, 16(1), 015028.
Bai, X., Gao, M., Syed, S., Zhuang, J., Xu, X., & Zhang, X. Q. (2018). Bioactive hydrogels for bone regeneration. Bioactive Materials, 3(4), 401–417.
Lee, W., & Park, J. (2016). 3D patterned stem cell differentiation using thermo-responsive methylcellulose hydrogel molds. Science and Reports, 6(1), 29408.
Cui, Z. K., Kim, S., Baljon, J. J., Wu, B. M., Aghaloo, T., & Lee, M. (2019). Microporous methacrylated glycol chitosan-montmorillonite nanocomposite hydrogel for bone tissue engineering. Nature Communications, 10(1), 3523.
Dong, L., Wang, S. J., Zhao, X. R., Zhu, Y. F., & Yu, J. K. (2017). 3D- Printed poly(ε-caprolactone) scaffold integrated with cell-laden chitosan hydrogels for bone tissue engineering. Science and Reports, 7(1), 13412.
Liu, S. Q., Tay, R., Khan, M., Rachel Ee, P. L., Hedrick, J. L., & Yang, Y. Y. (2010). Synthetic hydrogels for controlled stem cell differentiation. Soft Matter, 6(1), 67–81.
Martins, A. M., Alves, C. M., Kurtis Kasper, F., Mikos, A. G., & Reis, R. L. (2010). Responsive and situ-forming chitosan scaffolds for bone tissue engineering applications: An overview of the last decade. Journal of Materials Chemistry, 20(9), 1638–1645.
Tungmunnithum, D., Thongboonyou, A., Pholboon, A., & Yangsabai, A. (2018). Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines, 5(3), 93.
Lin, D., Xiao, M., Zhao, J., Li, Z., Xing, B., Li, X., Kong, M., Li, L., Zhang, Q., Liu, Y., Chen, H., Qin, W., Wu, H., & Chen, S. (2016). An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules, 21(10), 1374.
Venugopal, E., Rajeswaran, N., Sahanand, K. S., Bhattacharyya, A., & Rajendran, S. (2019). In vitro evaluation of phytochemical loaded electrospun gelatin nanofibers for application in bone and cartilage tissue engineering. Biomedical Materials, 14(1), 015004.
Zhang, Y., Yu, T., Peng, L., Sun, Q., Wei, Y., & Han, B. (2020). Advancements in hydrogel-based drug sustained release systems for bone tissue engineering. Frontiers in Pharmacology, 11, 622.
Choi, W. S., Shin, P. G., Lee, J. H., & Kim, G. D. (2012). The regulatory effect of veratric acid on no production in LPS-stimulated RAW264.7 Macrophage Cells. Cell. Immunol., 280(2), 164–170.
Shin, S., Jung, E., Kim, S., Lee, K. E., Youm, J. K., & Park, D. (2013). Antagonist effects of veratric acid against UVB-induced cell damages. Molecules, 18(5), 5405–5419.
Sruthi, R., Balagangadharan, K., & Selvamurugan, N. (2020). Polycaprolactone/ polyvinylpyrrolidone coaxial electrospun fibers containing veratric acid-loaded chitosan nanoparticles for bone regeneration. Colloids Surfaces B Biointerfaces, 193, 111110.
Fan, J. J., Cao, L. G., Wu, T., Wang, D. X., Jin, D., Jiang, S., Zhang, Z. Y., Bi, L., & Pei, G. X. (2011). The dose-effect of icariin on the proliferation and osteogenic differentiation of human bone mesenchymal stem cells. Molecules, 16(12), 10123–10133.
Hardcastle, A. C., Aucott, L., Reid, D. M., & Macdonald, H. M. (2011). Associations between dietary flavonoid intakes and bone health in a scottish population. Journal of Bone and Mineral Research, 26(5), 941–947.
Menon, A. H., Soundarya, S. P., Sanjay, V., Chandran, S. V., Balagangadharan, K., & Selvamurugan, N. (2018). Sustained release of chrysin from chitosan-based scaffolds promotes mesenchymal stem cell proliferation and osteoblast differentiation. Carbohydrate Polymers, 195, 356–367.
Costa-Pinto, A. R., Reis, R. L., & Neves, N. M. (2011). scaffolds based bone tissue engineering: The role of chitosan. Tissue Engineering. Part B, Reviews, 17(5), 331–347.
Keller, L., Regiel-Futyra, A., Gimeno, M., Eap, S., Mendoza, G., Andreu, V., Wagner, Q., Kyzioł, A., Sebastian, V., Stochel, G., Arruebo, M., & Benkirane-Jessel, N. (2017). Chitosan-based nanocomposites for the repair of bone defects. Nanomedicine: Nanotechnology, Biology and Medicine, 13(7), 2231–2240.
Lavanya, K., Chandran, S. V., Balagangadharan, K., & Selvamurugan, N. (2020). Temperature- and PH-responsive chitosan-based injectable hydrogels for bone tissue engineering. Materials Science and Engineering C, 111, 110862.
Murugan, C., Venkatesan, S., & Kannan, S. (2017). Cancer therapeutic proficiency of dual-targeted mesoporous silica nanocomposite endorses combination drug delivery. ACS Omega, 2(11), 7959–7975.
Lioubavina-Hack, N., Karring, T., Lynch, S. E., & Lindhe, J. (2005). Methyl cellulose gel obstructed bone formation by GBR: An experimental study in rats. Journal of Clinical Periodontology, 32(12), 1247–1253.
Kim, M. H., Kim, B. S., Park, H., Lee, J., & Park, W. H. (2018). Injectable methylcellulose hydrogel containing calcium phosphate nanoparticles for bone regeneration. International Journal of Biological Macromolecules, 109, 57–64.
Tak, W. S., Kim, D. J., & Ryu, S. C. (2018). Preparation and properties of hydroxyapatite/methylcellulose for bone graft. Journal of the Korean Ceramic Society, 55(2), 145–152.
Ghanaati, S., Barbeck, M., Hilbig, U., Hoffmann, C., Unger, R. E., Sader, R. A., Peters, F., & Kirkpatrick, C. J. (2011). An injectable bone substitute composed of beta-tricalcium phosphate granules, methylcellulose and hyaluronic acid inhibits connective tissue influx into its implantation bed in vivo. Acta Biomaterialia, 7(11), 4018–4028.
Hong, D., Fritz, A. J., Gordon, J. A., Tye, C. E., Boyd, J. R., Tracy, K. M., Frietze, S. E., Carr, F. E., Nickerson, J. A., Van Wijnen, A. J., Imbalzano, A. N., Zaidi, S. K., Lian, J. B., Stein, J. L., & Stein, G. S. (2019). RUNX1-dependent mechanisms in biological control and dysregulation in cancer. Journal of Cellular Physiology, 234(6), 8597–8609.
Shariatinia, Z., & Jalali, A. M. (2018). Chitosan-Based hydrogels: Preparation, properties and applications. International Journal of Biological Macromolecules, 115, 194–220.
Vishal Gupta, N., Shivakumar, H. G. (2012). Investigation of swelling behavior and mechanical properties of a ph-sensitive superporous hydrogel composite. Iranian Journal of Pharmaceutical Research. Spring, 11(2), 481–93.
He, F. (2011). Bradford Protein Assay. BIO-PROTOCOL, 101, 45. https://doi.org/10.21769/BioProtoc.45
Murugan, C., Rayappan, K., Thangam, R., Bhanumathi, R., Shanthi, K., Vivek, R., Thirumurugan, R., Bhattacharyya, A., Sivasubramanian, S., Gunasekaran, P., & Kannan, S. (2016). Combinatorial nanocarrier based drug delivery approach for amalgamation of anti-tumor agents in breast cancer cells: An improved nanomedicine strategy. Science and Reports, 6(1), 34053.
Leena, R. S., Vairamani, M., & Selvamurugan, N. (2017). Alginate/gelatin scaffolds incorporated with silibinin-loaded chitosan nanoparticles for bone formation in vitro. Colloids and Surfaces. B, Biointerfaces, 158, 308–318.
Gothard, D., Smith, E. L., Kanczler, J. M., Black, C. R., Wells, J. A., Roberts, C. A., White, L. J., Qutachi, O., Peto, H., Rashidi, H., Rojo, L., Stevens, M. M., El Haj, A. J., Rose, F. R. A. J., Shakesheff, K. M., & Oreffo, R. O. C. (2015). In Vivo assessment of bone regeneration in alginate/bone ECM hydrogels with incorporated skeletal stem cells and single growth factors. PLoS ONE, 10(12), e0145080.
Halgren, T. A., Murphy, R. B., Friesner, R. A., Beard, H. S., Frye, L. L., Pollard, W. T., & Banks, J. L. (2004). Glide: A new approach for rapid, accurate docking and scoring. 2. Enrichment Factors in Database Screening. Journal of Medicinal Chemistry, 47, 1750–1759.
Armiento, A. R., Hatt, L. P., Sanchez Rosenberg, G., Thompson, K., & Stoddart, M. J. (2020). Functional biomaterials for bone regeneration: A lesson in complex biology. Advanced Functional Materials, 30, 1909874.
O’Brien, F. J. (2011). Biomaterials & Scaffolds for tissue engineering. Materials Today, 14(3), 88–95.
Chen, S. H., Wang, X. L., Xie, X. H., Zheng, L. Z., Yao, D., Wang, D. P., Leng, Y., Zhang, G., & Qin, L. (2012). Comparative study of osteogenic potential of a composite scaffold incorporating either endogenous bone morphogenetic protein-2 or exogenous phytomolecule icaritin: An in vitro efficacy study. Acta Biomaterialia, 8(8), 3128–3137.
Hsu, Y. L., Chang, J. K., Tsai, C. H., Chien, T. T. C., & Kuo, P. L. (2007). Myricetin induces human osteoblast differentiation through bone morphogenetic protein-2/P38 mitogen-activated protein kinase pathway. Biochemical Pharmacology, 73(4), 504–514.
Morris, C., Thorpe, J., Ambrosio, L., & Santin, M. (2006). The soybean isoflavone genistein induces differentiation of MG63 human osteosarcoma osteoblasts. Journal of Nutrition, 136(5), 1166–1170.
Zhang, X., Zhou, C., Zha, X., Xu, Z., Li, L., Liu, Y., Xu, L., Cui, L., Xu, D., & Zhu, B. (2015). Apigenin promotes osteogenic differentiation of human mesenchymal stem cells through JNK and P38 MAPK pathways. Molecular and Cellular Biochemistry, 407(1–2), 41–50.
Kocak, F. Z., Talari, A. C. S., Yar, M., & Rehman, I. U. (2020). In-situ forming PH and thermosensitive injectable hydrogels to stimulate angiogenesis: Potential candidates for fast bone regeneration applications. International Journal of Molecular Sciences, 21(5), 1633.
Narayanan, V., & Sumathi, S. (2019). Preparation, characterization and in vitro biological study of silk fiber/methylcellulose composite for bone tissue engineering applications. Polymer Bulletin, 76(6), 2777–2800.
Carbinatto, F. M., de Castro, A. D., Evangelista, R. C., & Cury, B. S. F. (2014). Insights into the swelling process and drug release mechanisms from cross-linked pectin/high amylose starch matrices. Asian Journal of Pharmaceutical Sciences, 9(1), 27–34.
Wilson, C. J., Clegg, R. E., Leavesley, D. I., & Pearcy, M. J. (2005). Mediation of biomaterial–cell interactions by adsorbed proteins: A review. Tissue Engineering, 11(1–2), 1–18.
Boardman, S. J., Lad, R., Green, D. D., & Thornton, P. (2017). Chitosan hydrogels for targeted dye and protein adsorption. Journal of Applied Polymer Science, 134, 44846.
Bonetti, L., De Nardo, L., & Farè, S. (2021). Thermo-responsive methylcellulose hydrogels: From design to applications as smart biomaterials. Tissue Engineering Part B: Reviews, 27(5), 486–513.
Ding, J., Zhang, J., Li, J., Li, D., Xiao, C., Xiao, H., Yang, H., Zhuang, X., & Chen, X. (2019). Electrospun polymer biomaterials. Prsogress in Polymer Science., 90, 1–34.
Feng, X., Li, J., Zhang, X., Liu, T., Ding, J., & Chen, X. (2019). Electrospun polymer micro/nanofibers as pharmaceutical repositories for healthcare. Journal of Controlled Release, 302, 19–41.
Rambhia, K. J., & Ma, P. X. (2015). Controlled drug release for tissue engineering. Journal of Controlled Release, 219, 119–128.
Tanan, W., Panichpakdee, J., & Saengsuwan, S. (2019). Novel Biodegradable hydrogel based on natural polymers: Synthesis, characterization, swelling/reswelling and biodegradability. European Polymer Journal, 112, 678–687.
Acknowledgements
All the authors were grateful to the authorities of their institutes and Researchers Supporting Project number (RSP-2023-R20), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Conceptualization, DK, BB, AMK, and WL; data curation, DK, BB, KP, VA; formal analysis and methodology, DK, KP, VA; formal analysis and bioinformatics, ME; writing—original draft preparation, BB and DK; data analysis and interpretation, NAA, WL, AMK; writing—review and editing, BB, MVA, NAA, SP, UI, VA, AMK, and WL; recourses and validation WL, BB. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
The authors gave their consent for publication.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Balamuralikrishnan Balasubramanian equally contributed as a first author.
Highlights
• Veratric acid (VA) is a phenolic phytocompound as potential use for clinical use.
• The VA-loaded chitosan and methylcellulose hydrogels (CS/MC-VA) was prepared.
• The bioavailability and osteogenic efficacy were investigated with molecular modeling analysis.
• The CS/MC-VA showed no cytotoxic effects on to mouse mesenchymal stem cells.
• The CS/MC-VA showed potential osteogenic efficacy for bone regeneration.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Durairaj, K., Balasubramanian, B., Arumugam, V.A. et al. Biocompatibility of Veratric Acid–Encapsulated Chitosan/Methylcellulose Hydrogel: Biological Characterization, Osteogenic Efficiency with In Silico Molecular Modeling. Appl Biochem Biotechnol 195, 4429–4446 (2023). https://doi.org/10.1007/s12010-023-04311-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04311-5