Abstract
Mesenchymal stem cells (MSCs) have exhibited great potential as a regenerative medicine, and MSC-derived paracrine effects, mainly including the secretion of various bioactive factors, play critical roles in MSC-based therapies. MSC-derived serum-free conditioned medium (MSC-CM) is defined as the secretome of MSC-derived bioactive factors and is considered a new cell-free therapeutic agent for disease treatment. However, the MSC-CM used in previous studies was prepared by a nearly disposable method that the MSCs were discarded after preparing MSC-CM, and the preparation time was variable; simultaneously, the viability changes of MSCs after MSC-CM preparation are still unknown. Therefore, this study takes MenSCs as a research project and aims to explore the suitable period of sustainable MenSC-CM preparation rather than using a disposable method. As expected, our results confirmed that MenSC-CM improves viability of both naïve targeted cells and H2O2-injured targeted cells, and suggested that 36 h is suitable for sustainable MenSC-CM preparation in which the angiogenic factors almost reach to the peak. Simultaneously, the MenSCs used to prepare the MenSC-CM for 36 h also maintained preferable cell viability and could be sustainably used for further MenSC-CM preparation. Moreover, the in vivo results further confirmed the improvement of MenSC-CM on promoting skin wound healing. Consequently, our results not only provide support for optimizing MSC-CM sustainable preparation based on various MSCs but also promote the comprehensive application of MenSCs in the clinic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stem cell transplantation has provided a new alternative for the treatment of various refractory diseases, and mesenchymal stem cells (MSCs) are most widely used in basic research and clinical applications [1,2,3,4]. It is well known that MSC-derived paracrine effects and immunomodulation after transplantation can promote wound healing, improve disease symptoms, and bring hope to the patients with refractory disease [3, 5, 6]. Generally, the homing ability of MSCs guides them to migrate into injured sites; thus, local regulation of the immune response and promotion of tissue regeneration mainly depend on MSC secretion of various chemokines, cytokines, and growth factors [6, 7]. MSCs can differentiate into local components to participate in the reconstruction of injured sites, collectively contributing to the recovery of injured tissue and immune homeostasis [8]. During the past decade, menstrual blood-derived endometrial stem cells (MenSCs), as a typical kind of MSC, have exhibited distinct advantages, such as noninvasiveness, periodic acquisition from discarded menstrual blood samples, extensive and abundant resources, and autologous transplantation [9, 10]. To date, MenSC-based therapies have exhibited promising improvements in the treatment of heart failure, and serious intrauterine adhesion and COVID-19 in the clinic, and no safety problems have been reported [10].
However, because of the unique biological characteristics of MSC products (living cells), the donor source, preparation process, storage and transportation conditions, and strict quality control of MSC products have great impacts on cell viability, which partly limits the clinical application of MSCs [11]. Previous studies have confirmed that MSC-derived paracrine effects after transplantation, mainly including secretion of various cytokines and growth factors, play an important nutritional and immunomodulatory role in the improvement of diseases [1,2,3, 12, 13]. Generally, the preparation of MSC-derived biological factors is performed by collecting serum-free conditioned medium during MSC culture, and the MSC-derived serum-free conditioned medium is named MSC-CM and is defined as the secretome of MSC-derived bioactive factors, mainly including soluble proteins, nucleic acids, lipids, and extracellular vesicles (microvesicles and exosomes) [13]. MSC-CM has exhibited significant improvement in cell viability and proliferative and anti-apoptotic capacity, especially for the regeneration of injured cells and tissues [14, 15]. Our published studies have confirmed that MenSC-CM contains a large number of vascular growth-promoting factors and nutritional factors, such as angiogenin, epidermal growth factor (EGF), and vascular endothelial growth factor (VEGF), which can promote cell proliferation and migration in vitro and in vivo [16]. Currently, published reports have found that MenSC-CM can ameliorate pulmonary fibrosis by regulating ROS production, mtDNA injury, and NLRP3 inflammasome activation, providing a new approach for the treatment of fibrotic lung diseases [17]. MSC-CM also attenuated oxidative stress-induced injury in hepatocytes by downregulating miR-486-5p and upregulating PIM1, which might be associated with the inhibition of the TGF-β/Smad signaling pathway [18]. Moreover, a previous study showed that MSC-CM is capable of rebuilding injured skin tissue by promoting the regeneration of skin tissue-associated cells [19]. Therefore, MenSC-CM is promising for extensive application in disease and antiaging treatment and exhibits superior improvement in the field of medical beauty.
MSC-CM has been considered a new cell-free therapeutic agent for disease treatment and exhibits unique advantages when compared with the direct application of MSCs in the clinic [13, 20]: (1) MSC-CM application avoids the potential safety problems for recipients, mainly including the potential risks of immunogenicity, tumorigenicity, and infection during and after MSC transplantation; (2) the quality control of MSC-CM is easier to perform and achieve standardization, while the safety, efficacy, and pharmacokinetic parameters of MSC-CM can be tested through traditional biological agent evaluation; (3) both naïve MSCs and gene-edited MSCs can be used to produce MSC-CM on a grand scale based on practical requirements, providing abundant and stable sources for bioactive factors; and (4) the storage and transportation of MSC-CM is more convenient and economical than MSCs themselves, effectively promoting the clinical application of MSC-CM.
Although the therapeutic effect of MSC-CM has been confirmed by many studies, the MSC-CM used in those studies was prepared by a nearly disposable method, and the preparation time was different between studies [20,21,22]. Most importantly, the viability changes of MSCs and how MSCs were treated after MSC-CM preparation were not mentioned. Therefore, MenSCs were chosen as the topic of this research project, and the aim of this study was to explore the suitable period of sustainable preparation of MenSC-CM to allow MenSCs to be cultured to sustainably produce MenSC-CM rather than producing MenSC-CM by a disposable method. Simultaneously, the prepared MenSC-CM should contain the optimal types and concentrations of bioactive factors, which would lead to the best improvement for disease. These results will not only promote the comprehensive application of MenSCs in the clinic but also provide support for optimizing MSC-CM preparation based on various MSCs.
Materials and Methods
Cells and Animals
This study was approved by the Ethics Committee of the Xinxiang Medical University. MenSCs were kindly and freely provided by Zhongyuan Stem Cell Institute (Xinxiang, Henan, China), and the MenSCs used in this study satisfied the criterion of the international requirements of MSCs [16]. Generally, frozen passage 3 (P3) MenSCs (n = 3, respectively isolated from 3 health donors with informed consents) were conventionally recovered in cell culture flask in growth medium (DMEM (Corning, USA) + 10% FBS (Gibco, Australia) + 1% mixture of penicillin and streptomycin (P/S, Genevie, Beijing, China, 100 ×)) at the density of 1 × 104 cells/cm2. When the cells reached 80–90% confluence, the MenSCs were detached and subcultured into new cell flasks. P4 to P6 MenSCs were used for the following experiments. Additionally, the targeted cells used in this study, including 293 T cells (human embryonic kidney cells), KGN cells (human ovarian granulosa cells), BJ cells (human skin fibroblasts), and L02 cells (human hepatic cells) were kept in our lab. 293 T cells and BJ cells were cultured in growth medium (DMEM + 10% FBS + 1% P/S (100 ×)); KGN cells were cultured in growth medium (DMEM/F12 + 10% FBS + 1% P/S (100 ×)); L02 cells were cultured in growth medium (RPMI1640 + 10% FBS + 1% P/S (100 ×)). All the cells were cultured at 37℃ with 5% humidified CO2, and conventionally detached and subcultured to new flasks.
Six-to-eight-week-old female BALB/c nude mice were purchased from Vital River Laboratories (Beijing, China), and were bred and housed at a specific pathogen-free condition on a 12-h light–dark cycle. Handling of mice and experimental procedures were approved by the Animal Research Committee of Xinxiang Medical University according to the Chinese Council on Animal Care guidelines.
Preparation of MenSC-Conditioned Medium (MenSC-CM)
MenSCs were inoculated in a 75 cm2 cell culture flask and cultured until they reached 70 to 80% confluence; then, the cells were washed twice with PBS, and fresh DMEM was supplied. The cells were cultured for different times (12 h, 24 h, 36 h, 48 h, and 72 h), and the morphology of MenSCs was imaged under a microscope. The associated MenSC-CM samples were collected, filtered with a 0.22 μm strainer, and stored at −80℃ for subsequent experiments. The protein concentration of MenSC-CM was measured with a conventional BCA assay and further confirmed by conventional SDS–PAGE. Thereafter, the expression of angiogenic factors in MenSC-CM was determined by protein array assays.
CCK8 Assay
In the effect of MenSC-CM on the viability of targeted cells, the targeted cells (293 T cells, KGN cells, BJ cells, and L02 cells) were conventionally prepared and seeded into 96-well plates (200 μL of cell suspension per well) at a density of 1 × 104 cells/cm2 in growth medium. After overnight culture, the supernatant was discarded, and the plates were washed twice with PBS; then, MenSC-CM (200μL per well) prepared for different time durations was added. After culturing for 24 and 48 h, CCK8 solution was added to each well, and the plates were incubated at 37 °C for another 2 h. Then, the absorbance was determined at 450 nm by a microplate reader (SpectraMax® i3, Molecular Devices, USA).
In the effect of MenSC-CM preparation on the viability of MenSCs, the MenSCs used to prepare MenSC-CM for different time durations (12 h, 24 h, 36 h, 48 h, and 72 h) were digested and reseeded into 96-well plates (200 μL of cell suspension per well) at a density of 1 × 104 cells/cm2 in fresh growth medium, and the cells were cultured for 24 and 48 h. A CCK8 assay was used to examine cell viability.
In the effect of MenSC-CM on the viability of H2O2-injured BJ cells and L02 cells, the BJ cells and L02 cells were respectively treated with serum-free medium containing 1 mM and 0.075 mM H2O2 for 1 h; and then the supernatant was removed and replaced with MenSC-CM and serum-free medium for another 24 and 48 h of culture after being washed twice with PBS; then, a CCK8 assay was used to examine cell viability. Furthermore, the apoptosis and the expression of apoptotic proteins in H2O2-injured BJ cells and L02 cells with or without MenSC-CM treatment for 48 h were respectively determined by flow cytometry and western blot.
Protein Array Assays
A Quantibody ® Human Angiogenesis Array (QAH-ANG-1, RayBiotech, Norcross, GA, USA) was performed according to the manufacturer’s instructions to determine the concentration of 10 angiogenic factors (as shown in Fig. 2a) in tenfold-concentrated MenSC-CM by lyophilization (n = 3). Fluorescence signals were captured on glass chips using a laser scanner (InnoScan 300 Microarray Scanner; Innopsys, Carbonne, France), the fluorescence intensities were normalized to those of the internal positive controls, and the concentration of targeted proteins was calculated by real-time standard curves.
Western Blot
The protein samples of cells in each group were conventionally collected in RIPA lysis buffer containing protease inhibitor cocktail according to the manufacturer’s instructions (Beyotime, China), and the concentration of total protein was determined by BCA assay. Subsequently, the protein samples (10 μg per lane) were electrophoresed by 10–12% SDS–PAGE and transferred to a nitrocellulose membrane. After blocking with 5% nonfat milk in TBS containing 0.1% Tween 20, the membrane was incubated with primary antibodies (Table S1) overnight at 4 °C and then with HRP-conjugated secondary antibody for 2 h at room temperature. Finally, the expression of anti-apoptotic indicators (Bcl-2 and Bcl-xL), pro-apoptotic indicators (BAX), proliferative indicator (p-Akt and Akt), and autophagic indicators (LC3A/B, Beclin and p62) were developed using the ECL WB substrate kit and detected using a chemiluminescence instrument (Amersham Imager 600, GE Healthcare Life Sciences, USA), and the gray value of the targeted proteins was quantified by the ImageJ software. GAPDH was used as the internal control, and the expression level of the target proteins is indicated by the ratio to GAPDH.
Flow Cytometry
Apoptosis detection was performed using an Annexin V/PI staining kit (Beyotime, China). Briefly, 0.5 mL cell suspension (1 × 106cells/mL) was washed with PBS for twice and centrifuged at 1200 rpm for 5 min; after the supernatant was discarded, 210 μL working solution containing 5 μL Annexin V-FITC and 10 μL PI was used to gently re-suspend the cell sedimentation evenly. Next, the cell suspension was incubated at 4℃ for 20 min; then, the samples were diluted with PBS to the volume of 500 μL and filtered with 100 μm cell strainer for the final flow cytometry detection (FACS Calibur, BD, USA).
Wound Healing Assay In Vivo
The BALB/c nude mice (n = 10) were anesthetized with 2–3% isoflurane and maintained in an anesthetic state with 1.5–2% isoflurane, which allowed the mice to breathe spontaneously during the surgery. Subsequently, the circular wound area (1 cm in diameter) was cut off following the prepared pattern by a sterilized surgical scissor on the back of the mouse. Then, 200 μL tenfold-concentrated MenSC-CM was evenly applied to the wound area of the mice (n = 5) three times a day for the first 3 days; the wound area in the mice of control group (n = 5) received equal volume of tenfold-concentrated fresh DMEM. Images were captured to record wound healing within 14 days, and the wound area was quantified by the Image J software.
Statistical Analysis
All the data are representative of at least three independent experiments and presented as the mean ± standard deviation, and the GraphPad Prism8.0 software was used for statistical analysis. Student’s t-test was used for comparisons between two groups; one-way ANOVA followed by Dunnett’s test was used for comparisons among multiple (≥ 3) groups. P < 0.05 was considered to indicate statistical significance.
Result
MenSC-CM Treatment Significantly Improved the Viability of Targeted Cells
As shown in Fig. 1a, b, the increase in the protein concentration in MenSC-CM was time dependent, and the protein concentration in MenSC-CM reached its maximum value at 48 h. The subsequent viability assay indicated that MenSC-CM treatment was capable of promoting the viability of targeted cells, although the targeted cells exhibited different degrees of improvement after treatment with MenSC-CM prepared for different time durations (Fig. 1c–f).
MenSC-CM treatment significantly improved the viability of targeted cells. a The protein concentration of MenSC-CM prepared for different time durations was determined by BCA. b The protein samples of MenSC-CM prepared for different time durations were further analyzed by SDS–PAGE and Coomassie brilliant blue staining. c–f CCK8 assay was used to detect the viability improvement of targeted cells (293 T cells, BJ cells, L02 cells, and KGN cells) treated for 24 and 48 h with MenSC-CM prepared for different time durations
MenSC-CM Contains Abundant Angiogenic Factors
Consistent with the viability improvement of targeted cells treated with MenSC-CM, the subsequent protein assay confirmed that abundant biological factors are contained in MenSC-CM (Fig. 2). Simultaneously, we found that the concentration of angiogenic factors in MenSC-CM were also produced in a time-dependent manner, and although the concentrations of these bioactive factors in MenSC-CM increased with the extension of preparation time, most of these bioactive factors in MenSC-CM, mainly including angiogenin, bFGF, VEGF, HGF, and EGF, reached the desirable level when the preparation time was 36 h (Fig. 2).
The Preparation Time of MenSC-CM Significantly Affects MenSC Viability
To achieve sustainable preparation of MenSC-CM, the effect of preparation time of MenSC-CM on MenSC viability was examined. As shown in Fig. 3a, we clearly observed the morphological changes of MenSCs used to produce MenSC-CM. The morphology of MenSCs gradually became slim with the extension of preparation time, due to the cell stress response of resistance to adversity. Subsequently, the viability assay indicated that the MenSCs prepared with MenSC-CM for 24 h still exhibited superior proliferative capacity, but the proliferative capacity of MenSCs was significantly inhibited with the extension of preparation time. In particular, when the preparation time of MenSC-CM exceeded 48 h, the viability of MenSCs was almost impossible to recover (Fig. 3b). Consistent with the viability assay, the anti-apoptotic indicators (Bcl-2 and Bcl-xL) and proliferative indicator (Akt) in MenSCs were significantly upregulated with the extension of preparation time, and when the preparation time of MenSC-CM exceeded 36 h, the anti-apoptotic capacity of MenSCs was gradually impaired (Fig. 3c). Simultaneously, the autophagic activity of MenSCs used to produce MenSC-CM was gradually upregulated with the preparation time extended, which was indicated by the gradual upregulation of Beclin and LC3A/B-II, as well as the downregulation of p62. When the preparation time was extended to 48 h, the autophagic activity of MenSCs reached to peak; thereafter, the decrease in autophagic activity of MenSCs was observed with a longer MenSC-CM preparation time (Fig. 3d).
The preparation time of MenSC-CM significantly affected the viability of MenSCs. a Morphological changes in MenSCs used to prepare MenSC-CM for different time durations. b A CCK8 assay was used to examine the viability of MenSCs after preparing MenSC-CM for different time durations. c, d The expression of antiapoptotic and proliferative indictors and autophagy-associated proteins in MenSCs after preparing MenSC-CM for different time durations was examined by WB and quantified by the ImageJ software. *P < 0.05; **P < 0.01; NS, no statistical significance
MenSC-CM Significantly Improved the Viability of H2O2-Injured Cells in Vitro
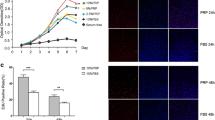
Based on the above results, MenSC-CM prepared for 36 h was selected for subsequent efficiency detection. As expected, MenSC-CM treatment significantly improved the viability of H2O2-injured BJ cells (Fig. 4a) and L02 cells (Fig. 4d) and effectively inhibited their apoptosis (Fig. 4c, f). This was likely due to upregulation of the expression of an anti-apoptotic indicator (Bcl-2) and a proliferative indicator (Akt) in targeted cells (Fig. 4b, e).
The viability improvement of H2O2-injured BJ cells and L02 cells treated with MenSC-CM. a, d The viability of H2O2-injured BJ cells and L02 cells treated with MenSC-CM was detected by CCK8 assay. b, e The expression of antiapoptotic and proliferative indictors in H2O2-injured BJ cells and L02 cells treated with MenSC-CM was determined by WB and quantified by the ImageJ software. c, f The apoptosis of H2O2-injured BJ cells and L02 cells treated with MenSC-CM was detected by flow cytometry and quantified by the FlowJo software. *P < 0.05, **P < 0.01; NS, no statistical significance
MenSC-CM Significantly Promotes the Skin Wound Healing In Vivo
Our results confirmed that MenSC-CM contains a large number of angiogenic and anti-inflammatory factors, which promote the regeneration of injured tissues. As expected, the in vivo results demonstrated that from day 4, the redness, swelling, and inflammatory exudation around the wound in MenSC-CM-treated mice were significantly relieved compared with those in the control group (Fig. 5b), and the healing rate of the wound area in MenSC-CM-treated mice was also significantly improved from day 7 (Fig. 5c).
Discussion
As complete secretome of MSC, MSC-CM contains a variety of cytokines that improve cell viability and is promising for extensive application in disease and antiaging treatments, which is regarded as an important supplement, and can contribute to the extensive application of MSCs in the clinic [20, 21, 23]. However, the MSC-CM used in published reports was prepared by a nearly disposable method, and the preparation time was uncertain, ranging from approximately 24 to 72 h. Most importantly, the viability changes of MSCs and how MSCs were treated after MSC-CM preparation were not described [22, 24, 25]. Furthermore, animal-derived ingredients are not permitted for use in the clinic due to their potential immunogenicity, but both bovine serum and serum substitute with unknown components are extensively used as the basic and core additives of MSC culture medium, and this generates increased complexity and uncertainty of the results of those studies [26, 27]. Therefore, serum-free MSC-CM has exhibited a wide range of applications from disease treatment to medical beauty. MenSCs, a promising stem cell alternative, have exhibited superior therapeutic effects in disease treatment and tissue regeneration, especially in the treatment of critically ill patients with COVID-19 [10]. MenSC-CM, defined as the secretome of MenSC-derived bioactive factors, is reasonably considered a promising supplement for the direct clinical application of MenSCs.
We reasonably speculate that the preparation time of MenSC-CM is positively correlated with both the secretion of bioactive factors and metabolic waste. Generally, a shorter preparation time will result in insufficient collection of cytokines in MenSC-CM, which directly impairs its therapeutic effects; however, a longer preparation time will not only cause the accumulation of metabolic waste in MenSC-CM but also significantly affect the viability of MenSCs, which is detrimental to therapeutic value of MenSC-CM and to the sustainable application of MenSCs. Therefore, the purpose of this study was to explore the suitable preparation time of MenSC-CM with optimal therapeutic effects. The study also aimed to maintain the viability of MenSCs, allowing them to sustainably produce MenSC-CM rather than producing MenSC-CM by a disposable method that results in the loss of stem cells.
Therefore, four types of cells isolated from various tissues were chosen as targeted cells to comprehensively evaluate the therapeutic potential of MenSC-CM. As expected, MenSC-CM prepared for all time durations improved the viability of targeted cells, which strongly suggested the improvement of MenSC-CM on various diseases [15, 17, 22]. Although the protein concentration in MenSC-CM prepared for 48 h nearly reached the maximum value, the subsequent protein assay confirmed that the expression of proangiogenic factors reached close to the maximum value in MenSC-CM prepared for 36 h, which provides support for the application of MSC-CM prepared from 24 to 48 h in previous reports [15, 21]. Subsequently, MenSC-CM prepared for 36 h not only significantly enhanced the anti-apoptotic capacity and improved the cell viability of H2O2-injured targeted cells in vitro but also promoted skin wound healing in vivo. The potential mechanism of promoting skin wound healing is likely due to the abundance of proangiogenic factors in MenSC-CM, such as angiogenin, bFGF, and VEGF, which play critical roles in the regeneration of blood vessels [28, 29].
Additionally, the stem cells were starved during the preparation of serum-free MenSC-CM, and the starvation state is known to activate intracellular autophagy, which exhibits a double-edged influence on the viability of stem cells [30]. Generally, when cells are treated with undernutrition, the lower level of intracellular ATP can activate the AMPK/SKP2/CARM1 signaling pathway and further activate intracellular autophagy, resulting in an increase in ATP and various cytokines to protect the cells against adversity [31]. However, long-term starvation causes continuous autophagy activation, which subsequently upregulates pro-apoptotic proteins and accelerates the formation of auto-phagosomes, finally inducing cell apoptosis by excessive autophagy activation [32]. Consistently, our results confirmed that short-term starvation (preparing MenSC-CM for less than 48 h) can significantly and continually activate the intracellular autophagy of stem cells and enhance their anti-apoptotic capacity by promoting the abundant secretion of substantial cytokines and further promoting cell viability after being re-subcultured. Simultaneously, long-term starvation (72 h) will induce excessive autophagy activation and impair the viability of stem cells used to prepare MenSC-CM by upregulating the expression of pro-apoptotic proteins.
In summary, because of its unique biological and physicochemical characteristics, MSC-CM is regarded as an important supplement and contributes to the extensive application of MSCs in the clinic. To sustainably obtain MSC-CM, rather than producing MSC-CM by a disposable method, this study taken MenSCs as a representative, and the results suggested that 36 h was the suitable time for MenSC-CM preparation. Besides the quality assurance of MenSC-CM prepared for 36 h, the MenSCs used to prepare the MenSC-CM also maintained their viability and could be sustainably used for further MenSC-CM preparation.
Next, the following research direction will be highlighted: (1) exploration: to explicit the components (proteins, lipids, polysaccharides, and nucleic acids) and existing delivery forms (exosomes and microvesicles) of associated bioactive factors in MenSC-CM and further explore their therapeutic effects and potential mechanisms for disease improvement and tissue regeneration. (2) Modification: gene engineering technology will be used not only to enhance the secretion of key bioactive factors in gene-modified MenSCs but also to establish immortalized MenSC cell lines with stable characteristics of MSC, which provide solid support for the efficiency and economy of MenSC-CM. Simultaneously, pretreatment of naïve MenSCs with physical microenvironment (hypoxia, hypothermia) or specific regents (cytokines, chemical compounds) could produce customized MenSC-CM with targeted therapeutic effect for different diseases. Additionally, combination of MenSC-CM with various biomaterials will realize the controlled release and targeted delivery of bioactive factors contained in MenSC-CM into the injured tissue, which significantly enhance their performance. (3) Application: besides being used in promoting wound healing (diabetic foot, bedsore, and burn wound), MenSC-CM could be applied in a variety of diseases treatment, including ophthalmic diseases (in the form of eyedrops), respiratory diseases (in the form of atomization), and skin diseases (in the form of ointments and creams). Simultaneously, MenSC-CM also exhibit promising application in the field of medical beauty. Furthermore, under the premise of sustainable production of MenSC-CM, optimizing the parameters, such as dose, delivery route, and time window, will accelerate the application of MenSC-CM and ensure their therapeutic effects.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Yu, H., Huang, Y., & Yang, L. (2022). Research progress in the use of mesenchymal stem cells and their derived exosomes in the treatment of osteoarthritis. Ageing research reviews, 80, 101684.
Tang, Y., Wu, P., Li, L., Xu, W., & Jiang, J. (2022). Mesenchymal stem cells and their small extracellular vesicles as crucial immunological efficacy for hepatic diseases. Frontiers in immunology, 13, 880523.
Jayaramayya, K., Mahalaxmi, I., Subramaniam, M. D., Raj, N., Dayem, A. A., Lim, K. M., Kim, S. J., An, J. Y., Lee, Y., Choi, Y., Raj, A., Cho, S. G., & Vellingiri, B. (2020). Immunomodulatory effect of mesenchymal stem cells and mesenchymal stem-cell-derived exosomes for COVID-19 treatment. BMB reports, 53(8), 400–412.
Li, N., & Hua, J. (2017). Interactions between mesenchymal stem cells and the immune system. Cellular and molecular life sciences, 74(13), 2345–2360.
Lo Nigro, A., Gallo, A., Bulati, M., Vitale, G., Paini, D. S., Pampalone, M., Galvagno, D., Conaldi, P. G., & Miceli, V. (2021). Amnion-derived mesenchymal stromal/stem cell paracrine signals potentiate human liver organoid differentiation: Translational implications for liver regeneration. Frontiers in medicine, 8, 746298.
Pankajakshan, D., & Agrawal, D. K. (2014). Mesenchymal stem cell paracrine factors in vascular repair and regeneration. Journal of biomedical technology and research, 1(1), 10.
Liesveld, J. L., Sharma, N., & Aljitawi, O. S. (2020). Stem cell homing: From physiology to therapeutics. Stem cells, 38(10), 1241–1253.
Rashid, S., Salim, A., Qazi, R.-E.-M., Malick, T. S., & Haneef, K. (2022). Sodium butyrate induces hepatic differentiation of mesenchymal stem cells in 3D collagen scaffolds. Applied biochemistry and biotechnology, 194(8), 3721–3732.
Chen, L., Qu, J., Cheng, T., Chen, X., & Xiang, C. (2019). Menstrual blood-derived stem cells: Toward therapeutic mechanisms, novel strategies, and future perspectives in the treatment of diseases. Stem cell research & therapy, 10(1), 406.
Xu, X., Jiang, W., Chen, L., Xu, Z., Zhang, Q., Zhu, M., Ye, P., Li, H., Yu, L., Zhou, X., Zhou, C., Chen, X., Zheng, X., Xu, K., Cai, H., Zheng, S., Jiang, W., Wu, X., Li, D., Chen, L., … Li, L. (2021). Evaluation of the safety and efficacy of using human menstrual blood-derived mesenchymal stromal cells in treating severe and critically ill COVID-19 patients: An exploratory clinical trial. Clinical and translational medicine, 11(2), e297.
Zhao, Q., Han, Z., Wang, J., & Han, Z. (2021). Development and investigational new drug application of mesenchymal stem/stromal cells products in China. Stem cells translational medicine, 10(Suppl 2), S18–S30.
Harrell, C. R., Fellabaum, C., Jovicic, N., et al. (2019). Molecular mechanisms responsible for therapeutic potential of mesenchymal stem cell-derived secretome. Cells, 8(5), 467.
Ahangar, P., Mills, S. J., & Cowin, A. J. (2020). Mesenchymal stem cell secretome as an emerging cell-free alternative for improving wound repair. International journal of molecular sciences, 21(19), 7038.
Mathew, B., Ravindran, et al. (2019). Mesenchymal stem cell-derived extracellular vesicles and retinal ischemia-reperfusion. Biomaterials, 197, 146–160.
Fu, X., Zhang, S., Li, T., Zhang, R., Lu, Y., Cheng, H., Xu, Y., Qin, H., Liu, Y., & Lin, J. (2022). Menstrual blood-derived endometrial stem cells ameliorate the viability of ovarian granulosa cells injured by cisplatin through activating autophagy. Reproductive toxicology, 110, 39–48.
Liu, Y., Niu, R., Yang, F., Yan, Y., Liang, S., Sun, Y., Shen, P., & Lin, J. (2018). Biological characteristics of human menstrual blood-derived endometrial stem cells. Journal of cellular and molecular medicine, 22(3), 1627–1639.
Sun, L., Zhu, M., Feng, W., Lin, Y., Yin, J., Jin, J., & Wang, Y. (2019). Exosomal miRNA let-7 from menstrual blood-derived endometrial stem cells alleviates pulmonary fibrosis through regulating mitochondrial DNA damage. Oxidative medicine and cellular longevity, 2019, 4506303.
Jun, E. K., Zhang, et al. (2014). Hypoxic conditioned medium from human amniotic fluid-derived mesenchymal stem cells accelerates skin wound healing through TGF-β/SMAD2 and PI3K/Akt pathways. International journal of molecular sciences, 15(1), 605–628.
Chen, W., Sun, Y., Gu, X., Cai, J., Liu, X., Zhang, X., Chen, J., Hao, Y., & Chen, S. (2021). Conditioned medium of human bone marrow-derived stem cells promotes tendon-bone healing of the rotator cuff in a rat model. Biomaterials, 271, 120714.
Praveen Kumar, L., Kandoi, S., Misra, R., Vijayalakshmi, S., Rajagopal, K., & Verma, R. S. (2019). The mesenchymal stem cell secretome: A new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine & growth factor reviews, 46, 1–9.
Lin, H., Chen, et al. (2021). Advances in mesenchymal stem cell conditioned medium-mediated periodontal tissue regeneration. Journal of translational medicine, 19(1), 456.
Li, H., Yahaya, B. H., Ng, W. H., Yusoff, N. M., & Lin, J. (2019). Conditioned medium of human menstrual blood-derived endometrial stem cells protects against MPP+-induced cytotoxicity in vitro. Frontiers in molecular neuroscience, 12, 80.
Giannasi, C., Niada, S., Della Morte, E., Casati, S., Orioli, M., Gualerzi, A., & Brini, A. T. (2021). Towards secretome standardization: Identifying key ingredients of MSC-derived therapeutic cocktail. Stem cells international, 2021, 3086122.
Cases-Perera, O., Blanco-Elices, C., Chato-Astrain, J., Miranda-Fernández, C., Campos, F., Crespo, P. V., Sánchez-Montesinos, I., Alaminos, M., Martín-Piedra, M. A., & Garzón, I. (2022). Development of secretome-based strategies to improve cell culture protocols in tissue engineering. Scientific reports, 12(1), 10003.
Xu, X., Wang, Y., Zhang, B., Lan, X., Lu, S., Sun, P., Li, X., Shi, G., Zhao, Y., Han, H., Du, C., & Wang, H. (2018). Treatment of experimental colitis by endometrial regenerative cells through regulation of B lymphocytes in mice. Stem cell research & therapy, 9(1), 146.
Bui, H., Nguyen, L. T., & Than, U. (2021). Influences of xeno-free media on mesenchymal stem cell expansion for clinical application. Tissue engineering and regenerative medicine, 18(1), 15–23.
Astori, G., Amati, E., Bambi, F., Bernardi, M., Chieregato, K., Schäfer, R., Sella, S., & Rodeghiero, F. (2016). Platelet lysate as a substitute for animal serum for the ex-vivo expansion of mesenchymal stem/stromal cells: Present and future. Stem cell research & therapy, 7(1), 93.
Ma, B., Wang, T., Li, J., & Wang, Q. (2022). Extracellular matrix derived from Wharton’s Jelly-derived mesenchymal stem cells promotes angiogenesis via integrin αVβ3/c-Myc/P300/VEGF. Stem cell research & therapy, 13(1), 327.
Sun, Y., Xiong, X., & Wang, X. (2020). The miR-590-3p/VEGFA axis modulates secretion of VEGFA from adipose-derived stem cells, which acts as a paracrine regulator of human dermal microvascular endothelial cell angiogenesis. Human cell, 33(3), 479–489.
Chang, Y. Y., Juhász, G., Goraksha-Hicks, P., Arsham, A. M., Mallin, D. R., Muller, L. K., & Neufeld, T. P. (2009). Nutrient-dependent regulation of autophagy through the target of rapamycin pathway. Biochemical Society transactions, 37(Pt 1), 232–236.
Shin, H. J., Kim, H., Oh, S., Lee, J. G., Kee, M., Ko, H. J., Kweon, M. N., Won, K. J., & Baek, S. H. (2016). AMPK-SKP2-CARM1 signalling cascade in transcriptional regulation of autophagy. Nature, 534(7608), 553–557.
Li, S., Li, H., Yang, D., Yu, X., Irwin, D. M., Niu, G., & Tan, H. (2017). Excessive autophagy activation and increased apoptosis are associated with palmitic acid-induced cardiomyocyte insulin resistance. Journal of diabetes research, 2017, 2376893.
Acknowledgements
We thank all members in our lab for their help in this article.
Funding
This study is supported by the Henan Province Foundation (No. 202300410307, No. 22A310008, No. 22A320043, No. 212102310611, and No. LHGJ20200939) and the Xinxiang City Foundation (Nos. GG2020009 and GG2021029)
Author information
Authors and Affiliations
Contributions
LR. S: conducted most of the experiments, writing—original draft.
RY. Z and YL. L: conducted cell viability analysis.
JX. Y: conducted the model of wound healing.
SH. Z, YL. S, and HB. C: conducted comprehensive analysis data.
YL. L: funding acquisition, investigation, project administration, supervision, writing—review and editing.
JT. L: provided experimental reagents and equipment, review & editing.
All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Competent authority approval was obtained from the Ethics Review Board of Xinxiang Medical University. Handling of mice and experimental procedures were approved by the Animal Research Committee of Xinxiang Medical University according to the Chinese Council on Animal Care guidelines.
Consent to Participate
Not applicable
Consent for Publication
Not applicable
Conflict of Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shang, L., Zhang, R., Yan, J. et al. Sustainable Production and Activity Determination of Serum-Free Conditioned Medium from Menstrual Blood-Derived Endometrial Stem Cells. Appl Biochem Biotechnol 195, 1109–1121 (2023). https://doi.org/10.1007/s12010-022-04205-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-04205-y