Abstract
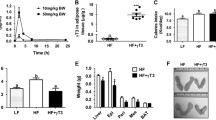
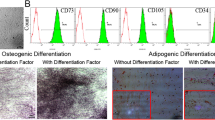
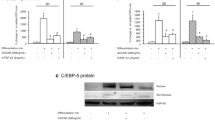
Type 2 diabetes mellitus (T2DM) adversely affects the essential characteristics of adipose tissue-derived mesenchymal stem cells (AdMSCs). Given that T2DM is associated with an altered serum free fatty acid (FFA) profile, we examined whether diabetic serum FFAs influence the viability, differentiation, and fatty acid composition of the major lipid fractions of human AdMSCs in vitro. Serum FFAs were isolated from 7 diabetic and 10 healthy nondiabetic female individuals. AdMSCs were cultured and differentiated into primordial germ cell-like cells (PGCLCs) in the presence of either diabetic or nondiabetic FFAs. Cell viability was assessed using trypan blue staining. Cell differentiation was evaluated by measuring the PGCLC transcriptional markers Blimp1 and Stella. Lipid fractionation and fatty acid quantification were performed using thin-layer chromatography and gas–liquid chromatography, respectively. Both diabetic and nondiabetic FFAs significantly reduced the viability of PGCLCs. The gene expression of both differentiation markers was significantly lower in cells exposed to diabetic FFAs than in those treated with nondiabetic FFAs. Saturated fatty acids were significantly increased and linoleic acid was significantly decreased in the cellular phospholipid fraction after exposure to diabetic FFAs. In contrast, monounsaturated fatty acids were reduced and linoleic acid was elevated in the cellular triglyceride fraction in response to diabetic FFAs. Such an altered serum FFA profile in patients with T2DM reduces the proliferation and differentiation potential of AdMSCs, presumably due to the aberrant distribution of fatty acids into cell phospholipids and triglycerides.
Graphical Abstract





Similar content being viewed by others
Data Availability Statement
Data will be available from corresponding author on reasonable request.
Abbreviations
- AdMSCs:
-
Adipose derived mesenchymal stem cells
- DM:
-
Diabetes mellitus
- DS:
-
Diabetic serum
- FBS:
-
Fasting blood sugar
- FFAs:
-
Free fatty acids
- MSCs:
-
Mesenchymal stem cells
- MUFAs:
-
Monounsaturated fatty acids
- nDS:
-
Non diabetic serum
- PUFAs:
-
Polyunsaturated fatty acids
- SFAs:
-
Saturated fatty acids
- PGCLC:
-
Primordial germ cell-like cell
References
Roden, M., & Shulman, G. I. (2019). The integrative biology of type 2 diabetes. Nature, 576(7785), 51–60.
Association, A. D. (2014). Diagnosis and classification of diabetes mellitus. Diabetes care, 37(Supplement_1), S81–S90.
Deshpande, A. D., Harris-Hayes, M., & Schootman, M. (2008). Epidemiology of diabetes and diabetes-related complications. Physical therapy, 88(11), 1254–1264.
Guariguata, L., et al. (2011). The International Diabetes Federation diabetes atlas methodology for estimating global and national prevalence of diabetes in adults. Diabetes research and clinical practice, 94(3), 322–332.
Thong, E. P., et al. (2020). Diabetes: A metabolic and reproductive disorder in women. The Lancet Diabetes & Endocrinology, 8(2), 134–149.
Vander Borght, M., & Wyns, C. (2018). Fertility and infertility: Definition and epidemiology. Clinical biochemistry, 62, 2–10.
Fayezi, S., et al. (2018). Primary Culture of Human Cumulus Cells Requires Stearoyl-Coenzyme A Desaturase 1 Activity for Steroidogenesis and Enhancing Oocyte In Vitro Maturation. Reproductive Sciences, 25(6), 844–853.
Cena, H., Chiovato, L., & Nappi, R. E. (2020). Obesity, polycystic ovary syndrome, and infertility: A new avenue for GLP-1 receptor agonists. The Journal of Clinical Endocrinology & Metabolism, 105(8), e2695–e2709.
Broughton, D. E., & Moley, K. H. (2017). Obesity and female infertility: Potential mediators of obesity’s impact. Fertility and sterility, 107(4), 840–847.
Jungheim, E. S., & Moley, K. H. (2010). Current knowledge of obesity’s effects in the pre-and periconceptional periods and avenues for future research. American journal of obstetrics and gynecology, 203(6), 525–530.
Mehdizadeh, A., et al. (2011). Correlation between the level of cholesteryl ester transfer protein in follicular fluid with fertilization rates in IVF/ ICSI cycles. Iranian Journal of Reproductive Medicine, 9(3), 193–198.
Baksh, D., Song, L., & Tuan, R. S. (2004). Adult mesenchymal stem cells: Characterization, differentiation, and application in cell and gene therapy. Journal of cellular and molecular medicine, 8(3), 301–316.
Goenka, V., et al. (2020). Therapeutic potential of mesenchymal stem cells in treating both types of diabetes mellitus and associated diseases. Journal of Diabetes & Metabolic Disorders, 19(2), 1979–1993.
Horwitz, E. M., Andreef, M., & Frassoni, F. (2006). Mesenchymal stromal cells. Current opinion in hematology, 13(6), 419.
Kariminekoo, S., et al. (2016). Implications of mesenchymal stem cells in regenerative medicine. Artificial cells, nanomedicine, and biotechnology, 44(3), 749–757.
Smith, A. N., et al. (2012). Unsaturated fatty acids induce mesenchymal stem cells to increase secretion of angiogenic mediators. Journal of cellular physiology, 227(9), 3225–3233.
Lupette, J., & Benning, C. (2020). Human health benefits of very-long-chain polyunsaturated fatty acids from microalgae. Biochimie, 178, 15–25.
Hirano, T. (2018). Pathophysiology of diabetic dyslipidemia. Journal of Atherosclerosis and Thrombosis, 25(9), 771–782.
Seigneur, M., et al. (1994). Serum fatty acid profiles in type I and type II diabetes: Metabolic alterations of fatty acids of the main serum lipids. Diabetes research and clinical practice, 23(3), 169–177.
Hosseini, V., et al. (2021). A small molecule modulating monounsaturated fatty acids and Wnt signaling confers maintenance to induced pluripotent stem cells against endodermal differentiation. Stem Cell Research & Therapy, 12(1), 550.
Sessler, A. M., & Ntambi, J. M. (1998). Polyunsaturated fatty acid regulation of gene expression. The Journal of nutrition, 128(6), 923–926.
Fayyazpour, P., et al. (2022). Fatty acids of type 2 diabetic serum decrease the stemness properties of human adipose derived mesenchymal stem cells. Journal of Cellular Biochemistry, 123(7), 1157–1170.
Kim, K., et al. (2019). Associations between preconception plasma fatty acids and pregnancy outcomes. Epidemiology (Cambridge, Mass.), 30(Suppl 2), S37.
Zarezadeh, R., et al. (2021). Fatty acids of follicular fluid phospholipids and triglycerides display distinct association with IVF outcomes. Reproductive BioMedicine Online, 42(2), 301–309.
Maharlouei, N., et al. (2021). Prevalence and pattern of infertility in Iran: A systematic review and meta-analysis study. Women’s Health Bulletin, 8(2), 63–71.
Bligh, E. G., & Dyer, W. J. (1959). A rapid method of total lipid extraction and purification. Canadian journal of biochemistry and physiology, 37(8), 911–917.
Alizadeh, E., et al. (2016). The effect of dimethyl sulfoxide on hepatic differentiation of mesenchymal stem cells. Artificial cells, nanomedicine, and biotechnology, 44(1), 157–164.
Shirzeily, M. H., et al. (2013). Comparison of differentiation potential of male mouse adipose tissue and bone marrow derived-mesenchymal stem cells into germ cells. Iranian journal of reproductive medicine, 11(12), 965.
Lepage, G., & Roy, C. C. (1986). Direct transesterification of all classes of lipids in a one-step reaction. Journal of lipid research, 27(1), 114–120.
Qi, Y., et al. (2019). Applicability of adipose-derived mesenchymal stem cells in treatment of patients with type 2 diabetes. Stem cell research & therapy, 10(1), 1–13.
Horiguchi, M., et al. (2021). Characterizing the degeneration of nuclear membrane and mitochondria of adipose-derived mesenchymal stem cells from patients with type II diabetes. Journal of Cellular and Molecular Medicine, 25(9), 4298–4306.
Alicka, M., et al. (2019). Adipose-derived mesenchymal stem cells isolated from patients with type 2 diabetes show reduced “stemness” through an altered secretome profile, impaired anti-oxidative protection, and mitochondrial dynamics deterioration. Journal of Clinical Medicine, 8(6), 765.
Abu-Shahba, N., et al. (2021). Impact of type 2 diabetes mellitus on the immunoregulatory characteristics of adipose tissue-derived mesenchymal stem cells. The international journal of biochemistry & cell biology, 140, 106072.
Sobczak, A. I. S., Blindauer, C. A., & Stewart, A. J. (2019). Changes in plasma free fatty acids associated with type-2 diabetes. Nutrients, 11(9), 2022.
Ma, Y., et al. (2021). Potential biomarker in serum for predicting susceptibility to type 2 diabetes mellitus: Free fatty acid 22: 6. Journal of diabetes investigation, 12(6), 950–962.
Sobczak, A. I., et al. (2021). Lipidomic profiling of plasma free fatty acids in type-1 diabetes highlights specific changes in lipid metabolism. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids, 1866(1), 158823.
Chandra, K., et al. (2020). Effect of augmented glycation in mobilization of plasma free fatty acids in type 2 diabetes mellitus. Diabetes & Metabolic Syndrome: Clinical Research & Reviews, 14(5), 1385–1389.
Lu, J., et al. (2012). Palmitate causes endoplasmic reticulum stress and apoptosis in human mesenchymal stem cells: Prevention by AMPK activator. Endocrinology, 153(11), 5275–5284.
Gillet, C., et al. (2015). Oleate abrogates palmitate-induced lipotoxicity and proinflammatory response in human bone marrow-derived mesenchymal stem cells and osteoblastic cells. Endocrinology, 156(11), 4081–4093.
Yaghooti, H., Mohammadtaghvaei, N., & Mahboobnia, K. (2019). Effects of palmitate and astaxanthin on cell viability and proinflammatory characteristics of mesenchymal stem cells. International Immunopharmacology, 68, 164–170.
Qu, B., et al. (2018). MiR-449 overexpression inhibits osteogenic differentiation of bone marrow mesenchymal stem cells via suppressing Sirt1/Fra-1 pathway in high glucose and free fatty acids microenvironment. Biochemical and biophysical research communications, 496(1), 120–126.
Qu, B., et al. (2020). MiR-155 inhibition alleviates suppression of osteoblastic differentiation by high glucose and free fatty acids in human bone marrow stromal cells by upregulating SIRT1. Pflügers Archiv-European Journal of Physiology, 472(4), 473–480.
Kang, J. X., Wan, J. B., & He, C. (2014). Concise review: Regulation of stem cell proliferation and differentiation by essential fatty acids and their metabolites. Stem Cells, 32(5), 1092–1098.
Wiesenfeld, P. W., Babu, U. S., & O’Donnell, M. W. (2001). Effect of long-chain fatty acids in the culture medium on fatty acid composition of WEHI-3 and J774A. 1 cells. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 128(1), 123–134.
Huot, P. S., Sarkar, B., & Ma, D. W. (2010). Conjugated linoleic acid alters caveolae phospholipid fatty acid composition and decreases caveolin-1 expression in MCF-7 breast cancer cells. Nutrition research, 30(3), 179–185.
Zhang, C., et al. (2015). Growth inhibitory effect of polyunsaturated fatty acids (PUFAs) on colon cancer cells via their growth inhibitory metabolites and fatty acid composition changes. PLoS ONE, 10(4), e0123256.
Belal, S. A., et al. (2019). Effect of long chain fatty acids on triacylglycerol accumulation, fatty acid composition and related gene expression in primary cultured bovine satellite cells. Animal Biotechnology, 30(4), 323–331.
Weijers, R. N. (2015). Membrane flexibility, free fatty acids, and the onset of vascular and neurological lesions in type 2 diabetes. Journal of Diabetes & Metabolic Disorders, 15(1), 1–8.
Song, Y., & Jensen, M. D. (2021). Red blood cell triglycerides—a unique pool that incorporates plasma-free fatty acids and relates to metabolic health. Journal of Lipid Research, 62, 100131.
Funding
This work was supported by grants from Endocrine Research Center, Tabriz University of Medical Sciences and Tabriz University of Medical Sciences (numbers [59420] and [66852]) to AM. The last author is supported by fellowship programs from the Alexander von Humboldt Foundation. The graphical designs were created with BioRender.com.
Author information
Authors and Affiliations
Contributions
Conceiving the study and designing the experiments, SF and AM; acquisition of funding, critical review and commentary, MN; providing human samples, ZN; performing the experiments and drafting the manuscript, RZ; statistical analysis, AG; supervision and planning. All authors participated in editing and have read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
This study was approved by the Ethics Committee of Tabriz University of Medical Sciences (Code: IR.TBZMED.REC.1400.011).
Consent to Participate
Complete explanations of the study were given to the participants and informed consent was obtained.
Consent to Publish
The authors affirm that human research participants provided informed consent for publication of all Figures and Tables.
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
WHAT’S KNOWN?
• The serum free fatty acid profile is altered in type 2 diabetes.
WHAT’S NEW?
• The gene expression of both primordial germ cell-like cell differentiation markers significantly decreases in cells exposed to diabetic free fatty acids (FFAs).
• Diabetic FFAs significantly increase saturated fatty acids and decrease linoleic acid in the phospholipid fraction.
• Diabetic FFAs reduce monounsaturated fatty acids and increase linoleic acid in the triglyceride fraction.
Supplementary Information
Below is the link to the electronic supplementary material.
12010_2022_4204_MOESM1_ESM.docx
Supplementary file1 (DOCX 16 KB) Table S1. The primer sequences of the primordial germ cell-like cell differentiation markers.
12010_2022_4204_MOESM2_ESM.docx
Supplementary file2 (DOCX 14 KB) Table S2. Effects of diabetic and nondiabetic free fatty acids on the viability of primordial germ cell like cells.
12010_2022_4204_MOESM3_ESM.docx
Supplementary file3 (DOCX 14 KB) Table S3. Raw original data of fatty acid percentage or ratio of MSCs phospholipid and triglyceride fractions in the individually studied groups.

Figure S1
. Image of a thin-layer chromatography plate on which nondiabetic pooled whole serum (nDPWS) and lipid-depleted nondiabetic pooled serum (nDS) samples were run. The rectangles show the free fatty acid fractions in each serum sample.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Norouzi, Z., Zarezadeh, R., Mehdizadeh, A. et al. Free Fatty Acids from Type 2 Diabetes Mellitus Serum Remodel Mesenchymal Stem Cell Lipids, Hindering Differentiation into Primordial Germ Cells. Appl Biochem Biotechnol 195, 3011–3026 (2023). https://doi.org/10.1007/s12010-022-04204-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-04204-z