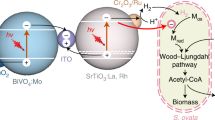
Abstract
Solar-driven biocatalysis technologies can combine inorganic photocatalytic materials with biological catalysts to convert CO2, light, and water into chemicals, offering the promise of high energy efficiency and a broader product scope than that of natural photosynthesis. Solar energy is the most abundant renewable energy source on earth, but it cannot be directly utilized by current industrial microorganisms. Therefore, the establishment of a solar-driven bio-catalysis platform, a bridge between solar energy and heterotrophic microorganisms, can dramatically increase carbon flux in biomanufacturing systems and consequently may revolutionize the biorefinery. This review first discusses the main applications of microbe-photocatalyst hybrid (MPH) systems in biorefinery processes. Then, various strategies to improve the electron transfer by microorganisms at the inorganic photocatalytic material interface are discussed, especially biohybrid systems based on autotrophic or heterotrophic bacteria and photocatalytic materials. Finally, we discuss the current challenges and offer potential solutions for the development of MPH systems.



Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Stephanopoulos, G. (2007). Challenges in engineering microbes for biofuels production. Science, 315(5813), 801–804. https://doi.org/10.1126/science.1139612
Kerr, R. A. (2007). Global warming is changing the world. Science, 316(5822), 188–190. https://doi.org/10.1126/science.316.5822.188
Su, L., & Ajo-Franklin, C. M. (2019). Reaching full potential: Bioelectrochemical systems for storing renewable energy in chemical bonds. Current Opinion in Biotechnology., 57, 66–72. https://doi.org/10.1016/j.copbio.2019.01.018
Sundstrom V, editor SOLAR Energy conversion - Natural to artificiaL. Conference on the NATO Advanced Study Institute on Bio-Photonics: Spectroscopy, Imaging, Sensing, and Manipulation; 2009 2011 Jul 02–17; Erice, ITALY2011.
Blankenship, R. E., Tiede, D. M., Barber, J., Brudvig, G. W., Fleming, G., Ghirardi, M., et al. (2011). Comparing photosynthetic and photovoltaic efficiencies and recognizing the potential for improvement. Science, 332(6031), 805–809. https://doi.org/10.1126/science.1200165
Zhu, X.-G., Long, S. P., & Ort, D. R. (2008). What is the maximum efficiency with which photosynthesis can convert solar energy into biomass? Current Opinion in Biotechnology., 19(2), 153–159. https://doi.org/10.1016/j.copbio.2008.02.004
Gassler, T., Sauer, M., Gasser, B., Egermeier, M., Troyer, C., Causon, T., et al. (2020). The industrial yeast Pichia pastoris is converted from a heterotroph into an autotroph capable of growth on CO2. Nature Biotechnology., 38(2), 210–216. https://doi.org/10.1038/s41587-019-0363-0
Xiao, K., Tsang, T. H., Sun, D., Liang, J., Zhao, H., Jiang, Z., et al. (2021). Interfacing iodine-doped hydrothermally carbonized carbon with Escherichia coli through an “Add-on” Mode for enhanced light-driven hydrogen production. Advanced Energy Materials, 11(21). https://doi.org/10.1002/aenm.202100291
Wei, W., Sun, P., Li, Z., Song, K., Su, W., Wang, B., et al. (2018). A surface-display biohybrid approach to light-driven hydrogen production in air. Science Advances, 4(2). https://doi.org/10.1126/sciadv.aap9253
Nichols, E. M., Gallagher, J. J., Liu, C., Su, Y., Resasco, J., Yu, Y., et al. (2015). Hybrid bioinorganic approach to solar-to-chemical conversion. Proceedings of the National Academy of Sciences of the United States of America., 112(37), 11461–11466. https://doi.org/10.1073/pnas.1508075112
Sahoo, P. C., Pant, D., Kumar, M., Puri, S. K., & Ramakumar, S. S. V. (2020). Material-microbe interfaces for solar-driven CO2 Bioelectrosynthesis. Trends in Biotechnology., 38(11), 1245–1261. https://doi.org/10.1016/j.tibtech.2020.03.008
Sakimoto, K. K., Zhang, S. J., & Yang, P. (2016). Cysteine-cystine photoregeneration for oxygenic photosynthesis of acetic acid from CO2 by a tandem inorganic-biological hybrid system. Nano Letters., 16(9), 5883–5887. https://doi.org/10.1021/acs.nanolett.6b02740
Zuo, W., Yu, Y., Huang, H. (2021). Making waves: Microbe-photocatalyst hybrids may provide new opportunities for treating heavy metal polluted wastewater. Water Research, 195. https://doi.org/10.1016/j.watres.2021.116984
Ma, H. H., Imran, M., Dang, Z., Hu, Z. (2018). Growth of metal halide perovskite, from nanocrystal to micron-scale crystal: A review. Crystals, 8(5). https://doi.org/10.3390/cryst8050182
Cheng, W.-H., Richter, M. H., May, M. M., Ohlmann, J., Lackner, D., Dimroth, F., et al. (2018). Monolithic photoelectrochemical device for direct water splitting with 19% Efficiency. Acs Energy Letters., 3(8), 1795–800. https://doi.org/10.1021/acsenergylett.8b00920
Zhou, X., Liu, R., Sun, K., Chen, Y., Verlage, E., Francis, S. A., et al. (2016). Solar-Driven reduction of 1 atm of CO2 to formate at 10% energy-conversion efficiency by use of a TiO2-protected III-V Tandem photoanode in conjunction with a bipolar membrane and a Pd/C cathode. Acs Energy Letters., 1(4), 764–770. https://doi.org/10.1021/acsenergylett.6b00317
Jia, J., Seitz, L. C., Benck, J. D., Huo, Y., Chen, Y., Ng, J. W. D, et al. (2016). Solar water splitting by photovoltaic-electrolysis with a solar-to-hydrogen efficiency over 30%. Nature Communications, 7. https://doi.org/10.1038/ncomms13237
LaBelle, E. V., Marshall, C. W., & May, H. D. (2020). Microbiome for the electrosynthesis of chemicals from carbon dioxide. Accounts of Chemical Research., 53(1), 62–71. https://doi.org/10.1021/acs.accounts.9b00522
Li, H., Opgenorth, P. H., Wernick, D. G., Rogers, S., Wu, T.-Y., Higashide, W., et al. (2012). Integrated Electromicrobial conversion of CO2 to higher alcohols. Science., 335(6076), 1596. https://doi.org/10.1126/science.1217643
Li, X., Sun, H., Mao, X., Lao, Y., & Chen, F. (2020). Enhanced photosynthesis of carotenoids in microalgae driven by light-harvesting gold nanoparticles. Acs Sustainable Chemistry & Engineering, 8(20), 7600–7608. https://doi.org/10.1021/acssuschemeng.0c00315
Forster, J., Famili, I., Palsson, B. O., & Nielsen, J. (2003). Large-scale evaluation of in silico gene deletions in Saccharomyces cerevisiae. Omics : A Journal of Integrative Biology, 7(2), 193–202. https://doi.org/10.1089/153623103322246584
Herrgard, M. J., Swainston, N., Dobson, P., Dunn, W. B., Arga, K. Y., Arvas, M., et al. (2008). A consensus yeast metabolic network reconstruction obtained from a community approach to systems biology. Nature Biotechnology, 26(10), 1155–1160. https://doi.org/10.1038/nbt1492
Guo, J., Suastegui, M., Sakimoto, K. K., Moody, V. M., Xiao, G., Nocera, D. G., et al. (2018). Light-driven fine chemical production in yeast biohybrids. Science, 362(6416), 813–816. https://doi.org/10.1126/science.aat9777
Fang, X., Kalathil, S., & Reisner, E. (2020). Semi-biological approaches to solar-to-chemical conversion. Chemical Society Reviews, 49(14), 4926–4952. https://doi.org/10.1039/c9cs00496c
Xiao, K., Liang, J., Wang, X., Hou, T., Ren, X., Yin, P., et al. (2022). Panoramic insights into semi-artificial photosynthesis: Origin, development, and future perspective. Energy & Environmental Science, 15(2), 529–549. https://doi.org/10.1039/d1ee03094a
Sakimoto, K. K., Kornienko, N., Cestellos-Blanco, S., Lim, J., Liu, C., & Yang, P. (2018). Physical biology of the materials-microorganism interface. Journal of the American Chemical Society, 140(6), 1978–1985. https://doi.org/10.1021/jacs.7b11135
Cestellos-Blanco, S., Kim, J. M., Watanabe, N. G., Chan, R. R., Yang, P. (2021). Molecular insights and future frontiers in cell photosensitization for solar-driven CO2 conversion. Iscience, 24(9). https://doi.org/10.1016/j.isci.2021.102952
Moreno-Garcia, L., Adjalle, K., Barnabe, S., & Raghavan, G. S. V. (2017). Microalgae biomass production for a biorefinery system: Recent advances and the way towards sustainability. Renewable & Sustainable Energy Reviews, 76, 493–506. https://doi.org/10.1016/j.rser.2017.03.024
Wei, L., Wang, Q., Xin, Y., Lu, Y., & Xu, J. (2017). Enhancing photosynthetic biomass productivity of industrial oleaginous microalgae by overexpression of RuBisCO activase. Algal Research-Biomass Biofuels and Bioproducts, 27, 366–375. https://doi.org/10.1016/j.algal.2017.07.023
de Mooij, T., Janssen, M., Cerezo-Chinarro, O., Mussgnug, J. H., Kruse, O., Ballottari, M., et al. (2015). Antenna size reduction as a strategy to increase biomass productivity: A great potential not yet realized. Journal of Applied Phycology, 27(3), 1063–1077. https://doi.org/10.1007/s10811-014-0427-y
Shin, W.-S., Lee, B., Jeong, B.-R., Chang, Y. K., & Kwon, J.-H. (2016). Truncated light-harvesting chlorophyll antenna size in Chlorella vulgaris improves biomass productivity. Journal of Applied Phycology, 28(6), 3193–202. https://doi.org/10.1007/s10811-016-0874-8
Wang, W., Yu, L.-J., Xu, C., Tomizaki, T., Zhao, S., Umena, Y., et al. (2019). Structural basis for blue-green light harvesting and energy dissipation in diatoms. Science, 363(6427), eaav0365. https://doi.org/10.1126/science.aav0365
Gronenberg, L. S., Marcheschi, R. J., & Liao, J. C. (2013). Next generation biofuel engineering in prokaryotes. Current Opinion in Chemical Biology, 17(3), 462–471. https://doi.org/10.1016/j.cbpa.2013.03.037
Cheng, S., Xing, D., Call, D. F., & Logan, B. E. (2009). Direct biological conversion of electrical current into methane by electromethanogenesis. Environmental Science & Technology, 43(10), 3953–3958. https://doi.org/10.1021/es803531g
Ye, J., Yu, J., Zhang, Y., Chen, M., Liu, X., Zhou, S., et al. (2019). Light-driven carbon dioxide reduction to methane by Methanosarcina barkeri-CdS biohybrid. Applied Catalysis B-Environmental, 257. https://doi.org/10.1016/j.apcatb.2019.117916
Sakimoto, K. K., Wong, A. B., & Yang, P. (2016). Self-photosensitization of nonphotosynthetic bacteria for solar-to-chemical production. Science, 351(6268), 74–77. https://doi.org/10.1126/science.aad3317
Kornienko, N., Sakimoto, K. K., Herlihy, D. M., Nguyen, S. C., Alivisatos, A. P., Harris, C. B., et al. (2016). Spectroscopic elucidation of energy transfer in hybrid inorganic-biological organisms for solar-to-chemical production. Proceedings of the National Academy of Sciences of the United States of America, 113(42), 11750–11755. https://doi.org/10.1073/pnas.1610554113
Zhang, R., He, Y., Yi, J., Zhang, L., Shen, C., Liu, S., et al. (2020). Proteomic and metabolic elucidation of solar-powered biomanufacturing by bio-abiotic hybrid system. Chem, 6(1), 234–249. https://doi.org/10.1016/j.chempr.2019.11.002
Jin, S., Jeon, Y., Jeon, M. S., Shin, J., Song, Y., Kang, S., et al. (2021). Acetogenic bacteria utilize light-driven electrons as an energy source for autotrophic growth. Proceedings of the National Academy of Sciences of the United States of America, 118(9). https://doi.org/10.1073/pnas.2020552118
Zhang, H., Liu, H., Tian, Z., Lu, D., Yu, Y., Cestellos-Blanco, S., et al. (2018). Bacteria photosensitized by intracellular gold nanoclusters for solar fuel production. Nature Nanotechnology, 13(10), 900–905. https://doi.org/10.1038/s41565-018-0267-z
Rowe, S. F., Le Gall, G., Ainsworth, E. V., Davies, J. A., Lockwood, C. W. J., Shi, L., et al. (2017). Light-driven H-2 evolution and C=C or C=O Bond hydrogenation by shewanella oneidensis: A versatile strategy for photocatalysis by nonphotosynthetic microorganisms. Acs Catalysis, 7(11), 7558–7566. https://doi.org/10.1021/acscatal.7b02736
Chen, M., Zhou, X.-F., Yu, Y.-Q., Liu, X., Zeng, R.J.-X., Zhou, S.-G., et al. (2019). Light-driven nitrous oxide production via autotrophic denitrification by self-photosensitized Thiobacillus denitrificans. Environment International, 127, 353–60. https://doi.org/10.1016/j.envint.2019.03.045
Xu, M., Tremblay, P.-L., Jiang, L., & Zhang, T. (2019). Stimulating bioplastic production with light energy by coupling Ralstonia eutropha with the photocatalyst graphitic carbon nitride. Green Chemistry, 21(9), 2392–2400. https://doi.org/10.1039/c8gc03695k
Tremblay, P-L., Xu, M., Chen, Y., Zhang, T. (2020). Nonmetallic abiotic-biological hybrid photocatalyst for visible water splitting and carbon dioxide reduction. Iscience, 23(1). https://doi.org/10.1016/j.isci.2019.100784
Venkidusamy, K., Megharaj, M., Schroeder, U., Karouta, F., Mohan, S. V., & Naidu, R. (2015). Electron transport through electrically conductive nanofilaments in Rhodopseudomonas palustris strain RP2. Rsc Advances, 5(122), 100790–100798. https://doi.org/10.1039/c5ra08742b
Huang, L., Liu, X., Zhang, Z., Ye, J., Rensing, C., Zhou, S., et al. (2022). Light-driven carbon dioxide reduction to methane by Methanosarcina barkeri in an electric syntrophic coculture. Isme Journal, 16(2), 370–377. https://doi.org/10.1038/s41396-021-01078-7
Guo, L., Ding, S., Liu, Y., Gao, C., Hu, G., Song, W., et al. (2022). Enhancing tryptophan production by balancing precursors in Escherichia coli. Biotechnology and Bioengineering, 119(3), 983–993. https://doi.org/10.1002/bit.28019
Yang, D., Park, S. Y., Park, Y. S., Eun, H., & Lee, S. Y. (2020). Metabolic engineering of Escherichia coli for natural product biosynthesis. Trends in Biotechnology, 38(7), 745–765. https://doi.org/10.1016/j.tibtech.2019.11.007
Qu, L., Xiu, X., Sun, G., Zhang, C., Yang, H., Liu, Y., et al. (2022). Engineered yeast for efficient de novo synthesis of 7-dehydrocholesterol. Biotechnology and Bioengineering, 119(5), 1278–1289. https://doi.org/10.1002/bit.28055
Wang, B., Zeng, C., Chu, K. H., Wu, D., Yip, H. Y., Ye, L., et al. (2017). Enhanced Biological hydrogen production from Escherichia coli with surface precipitated cadmium sulfide nanoparticles. Advanced Energy Materials, 7(20). https://doi.org/10.1002/aenm.201700611
Hu, G., Li, Z., Ma, D., Ye, C., Zhang, L., Gao, C., et al. (2021). Light-driven CO2 sequestration in Escherichia coli to achieve theoretical yield of chemicals. Nature Catalysis, 4(5), 395–406. https://doi.org/10.1038/s41929-021-00606-0
Dunleavy, R., Lu, L., Kiely, C. J., McIntosh, S., & Berger, B. W. (2016). Single-enzyme biomineralization of cadmium sulfide nanocrystals with controlled optical properties. Proceedings of the National Academy of Sciences of the United States of America, 113(19), 5275–5280. https://doi.org/10.1073/pnas.1523633113
Choi, Y., Park, T. J., Lee, D. C., & Lee, S. Y. (2018). Recombinant Escherichia coli as a biofactory for various single- and multi-element nanomaterials. Proceedings of the National Academy of Sciences of the United States of America, 115(23), 5944–5949. https://doi.org/10.1073/pnas.1804543115
Saldrnoto, K. K., Kornienko, N., & Yang, P. (2017). Cyborgian material design for solar fuel production: The emerging photosynthetic biohybrid systems. Accounts of Chemical Research, 50(3), 476–481. https://doi.org/10.1021/acs.accounts.6b00483
Hu, Z., Shen, Z., & Yu, J. C. (2017). Converting carbohydrates to carbon-based photocatalysts for environmental treatment. Environmental Science & Technology, 51(12), 7076–7083. https://doi.org/10.1021/acs.est.7b00118
Wang, X., Saba, T., Yiu, H. H. P., Howe, R. F., Anderson, J. A., & Shi, J. (2017). Cofactor NAD(P)H Regeneration inspired by heterogeneous pathways. Chem, 2(5), 621–654. https://doi.org/10.1016/j.chempr.2017.04.009
Suastegui, M., Ng, C. Y., Chowdhury, A., Sun, W., Cao, M., House, E., et al. (2017). Multilevel engineering of the upstream module of aromatic amino acid biosynthesis in Saccharomyces cerevisiae for high production of polymer and drug precursors. Metabolic Engineering, 42, 134–144. https://doi.org/10.1016/j.ymben.2017.06.008
Patakova, P., Linhova, M., Rychtera, M., Paulova, L., & Melzoch, K. (2013). Novel and neglected issues of acetone-butanol-ethanol (ABE) fermentation by clostridia: Clostridium metabolic diversity, tools for process mapping and continuous fermentation systems. Biotechnology Advances, 31(1), 58–67. https://doi.org/10.1016/j.biotechadv.2012.01.010
Wang, B., Chen, C., Jiang, Y., Ni, P., Zhang, C., Yang, Y., et al. (2021). Rational designing 0D/1D Z-scheme heterojunction on CdS nanorods for efficient visible-light-driven photocatalytic H-2 evolution. Chemical Engineering Journal, 412. https://doi.org/10.1016/j.cej.2021.128690
Zhao, Q., Wang, S., Lv, Z., Zupanic, A., Guo, S., Zhao, Q., et al. (2022). Using nanomaterials to increase the efficiency of chemical production in microbial cell factories: A comprehensive review. Biotechnology Advances, 59, 107982. https://doi.org/10.1016/j.biotechadv.2022.107982
Wang, X., Li, J., Zhang, C., Zhang, Y., Meng, J. (2021). Self-assembly of CdS@C. Beijerinckii hybrid system for efficient lignocellulosic butanol production. Chemical Engineering Journal, 424. https://doi.org/10.1016/j.cej.2021.130458
Jiang, Z., Wang, B., Yu, J. C., Wang, J., An, T., Zhao, H., et al. (2018). AglnS(2)/In2S3 heterostructure sensitization of Escherichia coli for sustainable hydrogen production. Nano Energy, 46, 234–240. https://doi.org/10.1016/j.nanoen.2018.02.001
Yu, Y., Wang, S., Teng, J., Zupanic, A., Guo, S., Tang, X., et al. (2022). Photocatalytic material-microbe hybrids: Applications in environmental remediations. Frontiers in Bioengineering and Biotechnology, 9. https://doi.org/10.3389/fbioe.2021.815181
Claassens, N. J., Sousa, D. Z., dos Santos, V. A. P. M., de Vos, W. M., & van der Oost, J. (2016). Harnessing the power of microbial autotrophy. Nature Reviews Microbiology, 14(11), 692–706. https://doi.org/10.1038/nrmicro.2016.130
Funding
This work was financially supported by the China Postdoctoral Science Foundation (2021M690081, 2021M691624).
Author information
Authors and Affiliations
Contributions
Na Wu: research literature, writing-original draft; Mingyan Xing: research literature, writing-original draft; Yingfeng Li and Qing Xu: resources, investigation; Ke Li: supervision, conceptualization, project administration.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable, because this is a review article does not include any experiments using human or animal participants.
Consent to Participate
Consent has been received from all the participating authors for this manuscript.
Consent for Publication
All the authors have provided their consent for publication.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, N., Xing, M., Li, Y. et al. Recent Advances In Microbe-Photocatalyst Hybrid Systems for Production of Bulk Chemicals: A Review. Appl Biochem Biotechnol 195, 1574–1588 (2023). https://doi.org/10.1007/s12010-022-04169-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-04169-z