Abstract
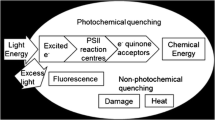
A major limitation in achieving high photosynthetic efficiency in microalgae mass cultures is the fact that the intensity of direct sunlight greatly exceeds the photosynthetic capacity of the cells. Due to the high pigment content of algal cells, the light absorption rate surpasses the much slower conversion rate to biochemical energy. The excess of light energy is predominantly dissipated as heat, decreasing the light use efficiency of the culture. Algae with a truncated antenna system could substantially increase biomass productivity of mass cultures because oversaturation of the photosystems and concomitant dissipation of light energy are minimized. In this study, we measured the areal biomass productivity of wild-type strain cultures and four promising antenna size mutant cultures of Chlamydomonas reinhardtii. This was performed under simulated mass culture conditions. The strains were cultivated in turbidostat controlled lab-scale panel photobioreactors at an incident light intensity of 1500 μmol photons m−2 s−1. The mutant cultures did not exhibit the expected higher productivity. The greatest mutant culture productivity values were approximate to those of the wild-type productivity of 1.9 g m−2 h−1. The high sensitivity to abrupt light shifts indicated that the mutant cultures experienced reduced fitness and higher susceptibility to photodamage. This can possibly be explained by impaired photoprotection mechanisms induced by the antenna complex alterations, or by unintended side effects of the genetic modifications. Still, if these effects could be eliminated, the principle of antenna size reduction is a promising strategy to increase productivity. Selection criteria for the future creation of antenna size mutants should, therefore, include tolerance to high light conditions.










Similar content being viewed by others
References
Alboresi A, Ballottari M, Hienerwadel R, Giacometti GM, Morosinotto T (2009) Antenna complexes protect photosystem I from photoinhibition. BMC Plant Biol 9:71
Blanken W, Cuaresma M, Wijffels RH, Janssen M (2013) Cultivation of microalgae on artificial light comes at a cost. Algal Res 2:333–340
Bonente G, Formighieri C, Mantelli M, Catalanotti C, Giuliano G, Morosinotto T, Bassi R (2011) Mutagenesis and phenotypic selection as a strategy toward domestication of Chlamydomonas reinhardtii strains for improved performance in photobioreactors. Photosynth Res 108:107–120
Cuaresma M, Janssen M, Vílchez C, Wijffels RH (2011) Horizontal or vertical photobioreactors? How to improve microalgae photosynthetic efficiency. Bioresource Technol 102:5129–5137
De Vitry C, Wollman F-A (1988) Changes in phosphorylation of thylakoid membrane proteins in light-harvesting complex mutants from Chlamydomonas reinhardtii. BBA-Bioenerg 933:444–449
Deblois CP, Marchand A, Juneau P (2013) Comparison of photoacclimation in twelve freshwater photoautotrophs (Chlorophyte, Bacillaryophyte, Cryptophyte and Cyanophyte) isolated from a natural community. PLoS One 8:e57139
Dubinsky Z, Falkowski PG, Wyman K (1986) Light harvesting and utilization by phytoplankton. Plant Cell Physiol 27:1335–1349
Duboc P, Marison I, Von Stockar U (1999) Quantitative calorimetry and biochemical engineering. Handb Therm Anal Calorim 4:267–365
Dye DJ (2010) Spatial light dilution as a technique for conversion of solar energy to algal biomass. Dissertation, Utah State University
Formighieri C, Franck F, Bassi R (2012) Regulation of the pigment optical density of an algal cell: filling the gap between photosynthetic productivity in the laboratory and in mass culture. J Biotechnol 162:115–123
Grewe S, Ballottari M, Alcocer M, D’Andrea C, Blifernez-Klassen O, Hankamer B, Mussgnug JH, Bassi R, Kruse O (2014) Light-harvesting complex protein LHCBM9 is critical for photosystem II activity and hydrogen production in Chlamydomonas reinhardtii. Plant Cell 26:1598–1611
Grossman AR, Bhaya D, Apt KE, Kehoe DM (1995) Light-harvesting complexes in oxygenic photosynthesis: diversity, control, and evolution. Annu Rev Genet 29:231–288
Huesemann MH, Hausmann TS, Bartha R, Aksoy M, Weissman JC, Benemann JR (2009) Biomass productivities in wild type and pigment mutant of Cyclotella sp. (Diatom). Appl Biochem Biotechnol 157:507–526
Hutner S, Provasoli L, Schatz A, Haskins C (1950) Some approaches to the study of the role of metals in the metabolism of microorganisms. Proc Am Philos Soc 94:152–170
Janssen M, Janssen M, de Winter M, Tramper J, Mur LR, Snel J, Wijffels RH (2000) Efficiency of light utilization of Chlamydomonas reinhardtii under medium-duration light/dark cycles. J Biotechnol 78:123–137
Jassby AD, Platt T (1976) Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Limnol Oceanogr 21:540–547
Kirst H, García-Cerdán JG, Zurbriggen A, Melis A (2012) Assembly of the light-harvesting chlorophyll antenna in the green alga Chlamydomonas reinhardtii requires expression of the TLA2-CpFTSY gene. Plant Physiol 158:930–945
Kliphuis AM, Klok AJ, Martens DE, Lamers PP, Janssen M, Wijffels RH (2012) Metabolic modeling of Chlamydomonas reinhardtii: energy requirements for photoautotrophic growth and maintenance. J Appl Phycol 24:253–266
Kwon J-H, Bernát G, Wagner H, Rögner M, Rexroth S (2013) Reduced light-harvesting antenna: consequences on cyanobacterial metabolism and photosynthetic productivity. Algal Res 2:188–195
Liberton M, Collins AM, Page LE, O’Dell WB, O’Neill H, Urban VS, Timlin JA, Pakrasi HB (2013) Probing the consequences of antenna modification in cyanobacteria. Photosynth Res 118:17–24
Melis A (1999) Photosystem-II damage and repair cycle in chloroplasts: what modulates the rate of photodamage in vivo? Trends Plant Sci 4:130–135
Melis A (2009) Solar energy conversion efficiencies in photosynthesis: minimizing the chlorophyll antennae to maximize efficiency. Plant Sci 177:272–280
Mussgnug JH, Thomas‐Hall S, Rupprecht J, Foo A, Klassen V, McDowall A, Schenk PM, Kruse O, Hankamer B (2007) Engineering photosynthetic light capture: impacts on improved solar energy to biomass conversion. Plant Biotechnol J 5:802–814
Nakajima Y, Ueda R (1998) Improvement of photosynthesis in dense microalgal suspension by reduction of light harvesting pigments. J Appl Phycol 9:503–510
Nakajima Y, Ueda R (2000) The effect of reducing light-harvesting pigment on marine microalgal productivity. J Appl Phycol 12:285–290
Nakajima Y, Tsuzuki M, Ueda R (2001) Improved productivity by reduction of the content of light-harvesting pigment in Chlamydomonas perigranulata. J Appl Phycol 13:95–101
Norsker N-H, Barbosa MJ, Vermuë MH, Wijffels RH (2011) Microalgal production—a close look at the economics. Biotechnol Adv 29:24–27
Oey M, Ross IL, Stephens E, Steinbeck J, Wolf J, Radzun KA, Kügler J, Ringsmuth AK, Kruse O, Hankamer B (2013) RNAi knock-down of LHCBM1, 2 and 3 increases photosynthetic H2 production efficiency of the green alga Chlamydomonas reinhardtii. PLoS One 8:e61375
Olive J, Wollman F-A, Bennoun P, Recouvreur M (1981) Ultrastructure of thylakoid membranes in C. reinhardtii: evidence for variations in the partition coefficient of the light-harvesting complex-containing particles upon membrane fracture. Arch Biochem Biophys 208:456–467
Ort DR, Zhu X, Melis A (2011) Optimizing antenna size to maximize photosynthetic efficiency. Plant Physiol 155:79–85
Peers G, Truong TB, Ostendorf E, Busch A, Elrad D, Grossman AR, Hippler M, Niyogi KK (2009) An ancient light-harvesting protein is critical for the regulation of algal photosynthesis. Nature 462:518–521
Perrine Z, Negi S, Sayre RT (2012) Optimization of photosynthetic light energy utilization by microalgae. Algal Res 1:134–142
Pirt S (1965) The maintenance energy of bacteria in growing cultures. Proc R Soc Lond B163:224–231
Polle JE, Kanakagiri S-D, Melis A (2003) tla1, a DNA insertional transformant of the green alga Chlamydomonas reinhardtii with a truncated light-harvesting chlorophyll antenna size. Planta 217:49–59
Takache H, Christophe G, Cornet JF, Pruvost J (2010) Experimental and theoretical assessment of maximum productivities for the microalgae Chlamydomonas reinhardtii in two different geometries of photobioreactors. Biotechnol Progr 26:431–440
Tanaka R, Tanaka A (2000) Chlorophyll b is not just an accessory pigment but a regulator of the photosynthetic antenna. Porphyrins 9:240–245
Tokutsu R, Minagawa J (2013) Energy-dissipative supercomplex of photosystem II associated with LHCSR3 in Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A 110:10016–10021
Tokutsu R, Iwai M, Minagawa J (2009) CP29, a monomeric light-harvesting complex II protein, is essential for state transitions in Chlamydomonas reinhardtii. J Biol Chem 284:7777–7782
Tredici MR, Zittelli GC (1998) Efficiency of sunlight utilization: tubular versus flat photobioreactors. Biotechnol Bioeng 57:187–197
Vejrazka C, Janssen M, Benvenuti G, Streefland M, Wijffels RH (2013) Photosynthetic efficiency and oxygen evolution of Chlamydomonas reinhardtii under continuous and flashing light. Appl Microbiol Biotechnol 97:1523–1532
Zemke PE, Sommerfeld MR, Hu Q (2013) Assessment of key biological and engineering design parameters for production of Chlorella zofingiensis (Chlorophyceae) in outdoor photobioreactors. Appl Microbiol Biotechnol 97:5645–5655
Acknowledgements
This work is part of the research programme of the Foundation for Fundamental Research on Matter (FOM) which is part of the Netherlands Organization for Scientific Research (NWO). This project was conducted within the research programme of BioSolar Cells, co-financed by the Dutch Ministry of Economic Affairs.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 130 kb)
Appendices
Appendix 1. Microalgae growth model and photobioreactor productivity
A simple kinetic model was constructed to describe microalgae productivity as a function of the light intensity. The model is based on two compartments. In the first compartment, the chloroplast, there is photosynthetic production of 3-carbon sugars (triose) symbolized by the 1-carbon sugar equivalent CH2O. This sugar production rate in the chloroplast (q c CH2O, molCH2O molx −1 s−1) is dependent upon light intensity I ph and described by the hyperbolic tangent model of Jassby and Platt (1976):
Where I ph is the PAR photon flux density (molph m−2 s−1), q c CH2O,m is the maximal sugar production rate (molCH2O molx −1 s−1) in the chloroplast, Y CH2O/ph,m is the maximal yield of sugar on light energy (molCH2O molph −1), and a x is the spectrally averaged optical cross section of the microalgae (m2 molx −1). a x can be calculated according to the following equation:
In which a x,λ is the optical cross section at wavelength λ. E n,λ (nm−1) represents the normalized spectral distribution of the light source (Fig. 2). It is the fraction of photons in the PAR region in a 1-nm interval at specific λ.
The cell minus the chloroplast comprises the second compartment in which the 3-carbon sugar is used to build new biomass at a specific growth rate μ. Another part of the sugar is respired in the mitochondria to provide energy (ATP) to support the growth reactions and to fulfill the maintenance requirements. The consumption of sugar in the chloroplast can be described by Pirt’s Law (Pirt 1965), resulting in the following relation:
and:
In these equations, m CH2O is the biomass specific maintenance rate (molCH2O molx −1 s−1), Y x/CH2O is the biomass yield on 3-carbon sugar (molx molCH2O −1), and μm is the biomass specific growth rate (s−1).
The biomass on yield on light energy (molx molph −1) can now be calculated as follows:
Here, q ph is the specific light absorption rate (molph molx −1 s−1), which is defined as follows:
The light intensity at which μ is equal to zero (i.e., where photosynthetic sugar production is compensated by maintenance-associated sugar consumption) is referred to as the photosynthetic compensation point (I ph,c). By numerical integration of Y x/ph from I ph = I ph,c to I ph = 1500 μmol photons m−2 s−1, the maximal biomass productivity per illuminated surface area (r x) is obtained in g m−2 h−1 (Eq. A7).
Appendix 2. Light intensity distribution over reactor surface
Appendix 3. Calculation of the extrapolated biomass concentration C x’ using the attenuation coefficient K x
For each mutant, the spectrally averaged attenuation coefficient (K x, m2 g−1) of the experiment with the darkest light regime (i.e., lowest I ph,out) was calculated. This attenuation coefficient K x was determined by measuring the light transmittance in the photobioreactor by measuring the I ph,in and I ph,out using a PAR light meter. This attenuation coefficient K x, therefore, also includes the effect of light scattering as it occurs in the photobioreactor. The coefficient K x is fundamentally different from the spectrally averaged optical cross section (a x) which only reflects true light absorption. K x was calculated using the equations indicated below in which d (m) is the light path of the photobioreactor.First, K x is calculated using Eq. C1 which was obtained by rearranging Eq. C2, Lambert-Beer’s law equation.
With K x known, C x’ at I ph,out = 10 μmol photons m−2 s−1 can be estimated using Eq. C3, which was also obtained by rearranging Eq. C2.
Rights and permissions
About this article
Cite this article
de Mooij, T., Janssen, M., Cerezo-Chinarro, O. et al. Antenna size reduction as a strategy to increase biomass productivity: a great potential not yet realized. J Appl Phycol 27, 1063–1077 (2015). https://doi.org/10.1007/s10811-014-0427-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-014-0427-y