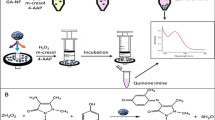
Abstract
Phenol, a pollutant frequently found in chemical industries effluents, is highly toxic even in low concentrations. This study reports a green, simple, and rapid method for qualitative phenol biosensing using horseradish peroxidase (HRP) hybrid nanoflowers made with copper (Cu2+-hNF) or calcium (Ca2+-hNF) ions. The enzyme was immobilized through protein-inorganic self-assembly into hybrid structures and subsequently supported onto a polyvinylidene fluoride (PVDF) membrane. SEM, EDS, FTIR, and XRD techniques sustained the effective enzyme encapsulation into hybrid structures. The protein concentration in the structures was 0.25 mg.mL−1 for both ions. The best temperature and pH were 60 °C and 7.4, respectively, for both hybrids and the free enzyme, suggesting that the immobilization did not affect the optimal conditions of the free HRP. Thermal stability from 25 to 70 °C and pH stability from 4.0 to 9.0 of the hybrids were also determined. Finally, using copper and calcium hybrids, both biosensors produced onto a PVDF membrane could detect phenol in concentrations ranging from 0.72 to 24.00 µmol.mL−1 in 1 min. In contrast, control biosensors produced with free enzyme have not presented a visible color change in the same conditions. The findings suggest a promising application of the developed biosensors in functional phenol detection.
Graphical abstract















Similar content being viewed by others
References
Li, X., Li, S., Liang, X., McClements, D. J., Liu, X., & Liu, F. (2020). Applications of oxidases in modification of food molecules and colloidal systems: Laccase, peroxidase and tyrosinase. Trends in Food Science and Technology, 103(March), 78–93.
Zhu, Y., Tao, H., Janaswamy, S., Zou, F., Cui, B., & Guo, L. (2021). The functionality of laccase- or peroxidase-treated potato flour: Role of interactions between protein and protein/starch. Food Chemistry, 341(February 2020), 128082.
BagheriPebdeni, A., & Hosseini, M. (2020). Fast and selective whole cell detection of Staphylococcus aureus bacteria in food samples by paper based colorimetric nanobiosensor using peroxidase-like catalytic activity of DNA-Au/Pt bimetallic nanoclusters. Microchemical Journal, 159(September), 105475.
Touahar, I. E., Haroune, L., Ba, S., Bellenger, J. P., & Cabana, H. (2014). Characterization of combined cross-linked enzyme aggregates from laccase, versatile peroxidase and glucose oxidase, and their utilization for the elimination of pharmaceuticals. Science of the Total Environment, 481, 90–99.
Poliakov, A. E., Dumshakova, A. V., Muginova, S. V., & Shekhovtsova, T. N. (2011). A peroxidase-based method for the determination of dopamine, adrenaline, and α-methyldopa in the presence of thyroid hormones in pharmaceutical forms. Talanta, 84(3), 710–716.
Na, S. Y., & Lee, Y. (2017). Elimination of trace organic contaminants during enhanced wastewater treatment with horseradish peroxidase/hydrogen peroxide (HRP/H2O2) catalytic process. Catalysis Today, 282, 86–94.
Al-Dhabi, N. A., Esmail, G. A., & Valan Arasu, M. (2021). Effective degradation of tetracycline by manganese peroxidase producing Bacillus velezensis strain Al-Dhabi 140 from Saudi Arabia using fibrous-bed reactor. Chemosphere, 268, 128726.
Yadav, N., Govindwar, S. P., Rane, N., Ahn, H.-J., Xiong, J.-Q., Jang, M., … Jeon, B.-H. (2021). Insights on the role of periphytic biofilm in synergism with Iris pseudacorus for removing mixture of pharmaceutical contaminants from wastewater.Journal of Hazardous Materials, 418(March), 126349.
Garg, S., Kumar, P., Singh, S., Yadav, A., Dumée, L. F., Sharma, R. S., & Mishra, V. (2020). Prosopis juliflora peroxidases for phenol remediation from industrial wastewater — An innovative practice for environmental sustainability. Environmental Technology and Innovation, 19, 100865.
Darwesh, O. M., Matter, I. A., & Eida, M. F. (2019). Development of peroxidase enzyme immobilized magnetic nanoparticles for bioremediation of textile wastewater dye. Journal of Environmental Chemical Engineering, 7(1), 102805.
de Oliveira, F. K., Santos, L. O., & Buffon, J. G. (2021). Mechanism of action, sources, and application of peroxidases. Food Research International, 143(March), 110266.
Sheldon, R. A., & van Pelt, S. (2013). Enzyme immobilisation in biocatalysis: Why, what and how. Chemical Society reviews, 42(15), 6223–6235.
Ge, J., Lei, J., & Zare, R. N. (2012). Protein-inorganic hybrid nanoflowers. Nature Nanotechnology, 7(7), 428–432.
Maurya, S. S., Nadar, S. S., & Rathod, V. K. (2020). Dual activity of laccase-lysine hybrid organic–inorganic nanoflowers for dye decolourization. Environmental Technology and Innovation, 19, 100798.
Wang, S., Ding, Y., Chen, R., Hu, M., Li, S., Zhai, Q., & Jiang, Y. (2018). Multilayer petal-like enzymatic-inorganic hybrid micro-spheres [CPO-(Cu/Co/Cd)3(PO4)2] with high bio-catalytic activity. Chemical Engineering Research and Design, 134(620), 52–61.
Rong, J., Zhang, T., Qiu, F., & Zhu, Y. (2017). Preparation of Efficient, stable, and reusable laccase-Cu 3 (PO 4) 2 hybrid microspheres based on copper foil for decoloration of Congo red. ACS Sustainable Chemistry and Engineering, 5(5), 4468–4477.
Patel, S. K. S., Otari, S. V., Li, J., Kim, D. R., Kim, S. C., Cho, B. K., … Lee, J. K. (2018). Synthesis of cross-linked protein-metal hybrid nanoflowers and its application in repeated batch decolorization of synthetic dyes. Journal of Hazardous Materials, 347, 442–450.
Wang, A., Chen, X., Yu, J., Li, N., Li, H., Yin, Y., … Wu, S. G. (2020). Green preparation of lipase@Ca3(PO4)2 hybrid nanoflowers using bone waste from food production for efficient synthesis of clindamycin palmitate. Journal of Industrial and Engineering Chemistry, 89, 383–391.
Zhang, Y., Sun, W., Elfeky, N. M., Wang, Y., Zhao, D., Zhou, H., …Bao, Y. (2020). Self-assembly of lipase hybrid nanoflowers with bifunctional Ca2+ for improved activity and stability. Enzyme and Microbial Technology, 132(2), 109408.
He, X., Chen, L., He, Q., Xiao, H., Zhou, X., & Ji, H. (2017). Cytochrome P450 enzyme-copper phosphate hybrid nano-flowers with superior catalytic performances for selective oxidation of sulfides. Chinese Journal of Chemistry, 35(5), 693–698.
Li, W. Y., Lu, S. Y., Bao, S. J., Shi, Z. Z., Lu, Z., Li, C. M., & Yu, L. (2018). Efficient in situ growth of enzyme-inorganic hybrids on paper strips for the visual detection of glucose. Biosensors and Bioelectronics, 99(August 2017), 603–611.
Cheon, H. J., Adhikari, M. D., Chung, M., Tran, T. D., Kim, J., & Kim, M. I. (2019). Magnetic nanoparticles-embedded enzyme-inorganic hybrid nanoflowers with enhanced peroxidase-like activity and substrate channeling for glucose biosensing. Advanced Healthcare Materials, 8(9), 1–8.
Batule, B. S., Park, K. S., Gautam, S., Cheon, H. J., Kim, M. I., & Park, H. G. (2019). Intrinsic peroxidase-like activity of sonochemically synthesized protein copper nanoflowers and its application for the sensitive detection of glucose. Sensors and Actuators, B: Chemical, 283(June 2018), 749–754.
Zhang, M., Yang, N., Liu, Y., & Tang, J. (2019). Synthesis of catalase-inorganic hybrid nanoflowers via sonication for colorimetric detection of hydrogen peroxide. Enzyme and Microbial Technology, 128(March), 22–25.
Altinkaynak, C., Yilmaz, I., Koksal, Z., Özdemir, H., Ocsoy, I., & Özdemir, N. (2016). Preparation of lactoperoxidase incorporated hybrid nanoflower and its excellent activity and stability. International Journal of Biological Macromolecules, 84, 402–409.
Ye, R., Zhu, C., Song, Y., Song, J., Fu, S., Lu, Q., … Lin, Y. (2016). One-pot bioinspired synthesis of all-inclusive protein-protein nanoflowers for point-of-care bioassay: Detection of: E. coli O157:H7 from milk. Nanoscale, 8(45), 18980–18986.
Gay, N. H., Phopin, K., Suwanjang, W., Songtawee, N., Ruankham, W., Wongchitrat, P., & Prachayasittikul, V. (2018). Neuroprotective effects of phenolic and carboxylic acids on oxidative stress-induced toxicity in human neuroblastoma SH-SY5Y cells. Neurochemical Research, 43(3), 619–636.
Jun, L. Y., Yon, L. S., Mubarak, N. M., Bing, C. H., Pan, S., Danquah, M. K., & Khalid, M. (2019). An overview of immobilized enzyme technologies for dye and phenolic removal from wastewater. Journal of Environmental Chemical Engineering, 7(2):102961
Arciuli, M., Palazzo, G., Gallone, A., & Mallardi, A. (2013). Bioactive paper platform for colorimetric phenols detection. Sensors and Actuators, B: Chemical, 186, 557–562.
Guan, Y., Liu, L., Chen, C., Kang, X., & Xie, Q. (2016). Effective immobilization of tyrosinase via enzyme catalytic polymerization of L-DOPA for highly sensitive phenol and atrazine sensing. Talanta, 160, 125–132.
Abdullah, J., Ahmad, M., Karuppiah, N., Heng, L. Y., & Sidek, H. (2006). Immobilization of tyrosinase in chitosan film for an optical detection of phenol. Sensors and Actuators, B: Chemical, 114(2), 604–609.
Hashim, H. S., Fen, Y. W., Sheh Omar, N. A., Abdullah, J., Daniyal, W. M. E. M. M., & Saleviter, S. (2020). Detection of phenol by incorporation of gold modified-enzyme based graphene oxide thin film with surface plasmon resonance technique. Optics Express, 28(7), 9738.
Wen, Y., Li, R., Liu, J., Zhang, X., Wang, P., Zhang, X., & Sun, B. (2020). Promotion effect of Zn on 2D bimetallic NiZn metal organic framework nanosheets for tyrosinase immobilization and ultrasensitive detection of phenol. Analytica Chimica Acta, 1127, 131–139.
Zhang, S., Zhao, H., & John, R. (2001). A dual-phase biosensing system for the determination of phenols in both aqueous and organic media. Analytica Chimica Acta, 441(1), 95–105.
Zhu, L., Gong, L., Zhang, Y., Wang, R., Ge, J., Liu, Z., & Zare, R. N. (2013). Rapid detection of phenol using a membrane containing laccase nanoflowers. Chemistry - An Asian Journal, 8(10), 2358–2360.
Casero, E., Petit-Domínguez, M. D., Vázquez, L., Ramírez-Asperilla, I., Parra-Alfambra, A. M., Pariente, F., & Lorenzo, E. (2013). Laccase biosensors based on different enzyme immobilization strategies for phenolic compounds determination. Talanta, 115, 401–408.
Roy, J. J., Abraham, T. E., Abhijith, K. S., Kumar, P. V. S., & Thakur, M. S. (2005). Biosensor for the determination of phenols based on Cross-Linked Enzyme Crystals (CLEC) of laccase. Biosensors and Bioelectronics, 21(1), 206–211.
Othman, A. M., & Wollenberger, U. (2020). Amperometric biosensor based on coupling aminated laccase to functionalized carbon nanotubes for phenolics detection. International Journal of Biological Macromolecules, 153, 855–864.
Jarosz-Wilkołazka, A., Ruzgas, T., & Gorton, L. (2005). Amperometric detection of mono- and diphenols at Cerrena unicolor laccase-modified graphite electrode: Correlation between sensitivity and substrate structure. Talanta, 66(5), 1219–1224.
Cho, S. H., Shim, J., Yun, S. H., & Moon, S. H. (2008). Enzyme-catalyzed conversion of phenol by using immobilized horseradish peroxidase (HRP) in a membraneless electrochemical reactor. Applied Catalysis A: General, 337(1), 66–72.
Rosatto, S. S., Sotomayor, P. T., Kubota, L. T., & Gushikem, Y. (2002). SiO2/Nb2O5 sol-gel as a support for HRP immobilization in biosensor preparation for phenol detection. Electrochimica Acta, 47(28), 4451–4458.
Lin, Z., Xiao, Y., Yin, Y., Hu, W., Liu, W., & Yang, H. (2014). Facile synthesis of enzyme-inorganic hybrid nanoflowers and its application as a colorimetric platform for visual detection of hydrogen peroxide and phenol. ACS Applied Materials and Interfaces, 6(13), 10775–10782.
Ozoner, S. K., Keskinler, B., & Erhan, E. (2011). HRP immobilized microporous Poly(styrene-divinylbenzene-polyglutaraldehyde) monolith for forced flow injected phenol biosensing. Materials Science and Engineering C, 31(3), 663–668.
Rahemi, V., Trashin, S., Hafideddine, Z., Meynen, V., Van Doorslaer, S., & De Wael, K. (2019). Enzymatic sensor for phenols based on titanium dioxide generating surface confined ROS after treatment with H2O2. Sensors and Actuators, B: Chemical, 283(December 2018), 343–348.
Çevik, E., Şenel, M., Baykal, A., & Abasiyanik, M. F. (2012). A novel amperometric phenol biosensor based on immobilized HRP on poly(glycidylmethacrylate)-grafted iron oxide nanoparticles for the determination of phenol derivatives. Sensors and Actuators, B: Chemical, 173, 396–405.
Yang, S., Chen, Z., Jin, X., & Lin, X. (2006). HRP biosensor based on sugar-lectin biospecific interactions for the determination of phenolic compounds. Electrochimica Acta, 52(1), 200–205.
Nadar, S. S., Gawas, S. D., & Rathod, V. K. (2016). Self-assembled organic–inorganic hybrid glucoamylase nanoflowers with enhanced activity and stability. International Journal of Biological Macromolecules, 92, 660–669.
Altinkaynak, C., Gulmez, C., Atakisi, O., & Özdemir, N. (2020). Evaluation of organic-inorganic hybrid nanoflower’s enzymatic activity in the presence of different metal ions and organic solvents. International Journal of Biological Macromolecules, 164, 162–171.
Talens-Perales, D., Fabra, M. J., Martínez-Argente, L., Marín-Navarro, J., & Polaina, J. (2020). Recyclable thermophilic hybrid protein-inorganic nanoflowers for the hydrolysis of milk lactose. International Journal of Biological Macromolecules, 151, 602–608.
Ghosh, K., Balog, E. R. M., Sista, P., Williams, D. J., Kelly, D., Martinez, J. S., & Rocha, R. C. (2014). Temperature-dependent morphology of hybrid nanoflowers from elastin-like polypeptides. APL Materials, 2(021101), 1–6
Trinder, P. (1969). Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Annals of Clinical Biochemistry: International Journal of Laboratory Medicine, 6(1), 24–27.
Malmstadt, H., & Hadjiioannou, T. (1963). Specific enzymatic determination of some alpha-amino acids by an automatic spectrophotometric reaction rate method. Analytical Chemistry, 35(1), 4–16.
Sun, J., Ge, J., Liu, W., Lan, M., Zhang, H., Wang, P., & Niu, Z. (2014). Multi-enzyme co-embedded organic-inorganic hybrid nanoflowers: Synthesis and application as a colorimetric sensor. Nanoscale, 6(1), 255–262.
Memon, A. H., Ding, R., Yuan, Q., Wei, Y., & Liang, H. (2019). Facile synthesis of alcalase-inorganic hybrid nanoflowers used for soy protein isolate hydrolysis to improve its functional properties. Food Chemistry, 289, 568–574.
Somturk, B., Yilmaz, I., Altinkaynak, C., Karatepe, A., Özdemir, N., & Ocsoy, I. (2016). Synthesis of urease hybrid nanoflowers and their enhanced catalytic properties. Enzyme and Microbial Technology, 86, 134–142.
Aydemir, D., Gecili, F., Özdemir, N., & Nuray Ulusu, N. (2020). Synthesis and characterization of a triple enzyme-inorganic hybrid nanoflower (TrpE@ihNF) as a combination of three pancreatic digestive enzymes amylase, protease and lipase. Journal of Bioscience and Bioengineering, 129(6), 679–686.
Jiang, W., Wang, X., Yang, J., Han, H., Li, Q., & Tang, J. (2018). Lipase-inorganic hybrid nanoflower constructed through biomimetic mineralization: A new support for biodiesel synthesis. Journal of Colloid and Interface Science, 514, 102–107.
Li, Y., Fei, X., Liang, L., Tian, J., Xu, L., Wang, X., & Wang, Y. (2016). The influence of synthesis conditions on enzymatic activity of enzyme-inorganic hybrid nanoflowers. Journal of Molecular Catalysis B: Enzymatic, 133, 92–97.
Yu, J., Wang, C., Wang, A., Li, N., Chen, X., Pei, X., … Wu, S. G. (2018). Dual-cycle immobilization to reuse both enzyme and support by reblossoming enzyme-inorganic hybrid nanoflowers. RSC Advances, 8(29), 16088–16094.
Ke, C., Fan, Y., Chen, Y., Xu, L., & Yan, Y. (2016). A new lipase-inorganic hybrid nanoflower with enhanced enzyme activity. RSC Advances, 6(23), 19413–19416.
Chung, M., Nguyen, T. L., Tran, T. Q. N., Yoon, H. H., Kim, I. T., & Kim, M. I. (2018). Ultrarapid sonochemical synthesis of enzyme-incorporated copper nanoflowers and their application to mediatorless glucose biofuel cell. Applied Surface Science, 429, 203–209.
Batule, B. S., Park, K. S., Kim, M. I., & Park, H. G. (2015). Ultrafast sonochemical synthesis of protein-inorganic nanoflowers. International Journal of Nanomedicine, 10, 137–142.
Gulmez, C., Altinkaynak, C., Özdemir, N., & Atakisi, O. (2018). Proteinase K hybrid nanoflowers (P-hNFs) as a novel nanobiocatalytic detergent additive. International Journal of Biological Macromolecules, 119, 803–810.
Koca, F. D., Demirezen Yilmaz, D., Ertas Onmaz, N., & Ocsoy, I. (2020). Peroxidase-like activity and antimicrobial properties of curcumin-inorganic hybrid nanostructure. Saudi Journal of Biological Sciences, 27(10), 2574–2579.
Ren, W., Li, Y., Wang, J., Li, L., Xu, L., Wu, Y., … Tian, J. (2019). Synthesis of magnetic nanoflower immobilized lipase and its continuous catalytic application. New Journal of Chemistry, 43(28), 11082–11090.
Ariza-Avidad, M., Salinas-Castillo, A., & Capitán-Vallvey, L. F. (2016). A 3D μPAD based on a multi-enzyme organic-inorganic hybrid nanoflower reactor. Biosensors and Bioelectronics, 77, 51–55.
He, G., Hu, W., & Li, C. M. (2015). Spontaneous interfacial reaction between metallic copper and PBS to form cupric phosphate nanoflower and its enzyme hybrid with enhanced activity. Colloids and Surfaces B: Biointerfaces, 135, 613–618.
Altinkaynak, C., Tavlasoglu, S., Kalin, R., Sadeghian, N., Ozdemir, H., Ocsoy, I., & Özdemir, N. (2017). A hierarchical assembly of flower-like hybrid Turkish black radish peroxidase-Cu2+ nanobiocatalyst and its effective use in dye decolorization. Chemosphere, 182, 122–128.
Alessi, A., Agnello, S., Buscarino, G., & Gelardi, F. M. (2013). Raman and IR investigation of silica nanoparticles structure. Journal of Non-Crystalline Solids, 362(1), 20–24.
Yu, Y., Fei, X., Tian, J., Xu, L., Wang, X., & Wang, Y. (2015). Self-assembled enzyme-inorganic hybrid nanoflowers and their application to enzyme purification. Colloids and Surfaces B: Biointerfaces, 130, 299–304.
Sun, X., Niu, H., Song, J., Jiang, D., Leng, J., Zhuang, W., … Ying, H. (2020). Preparation of a copper polyphosphate kinase hybrid nanoflower and its application in ADP regeneration from AMP. ACS Omega, 5(17), 9991–9998.
Cui, J., Zhao, Y., Liu, R., Zhong, C., & Jia, S. (2016). Surfactant-activated lipase hybrid nanoflowers with enhanced enzymatic performance. Scientific Reports, 6(29), 1–13.
Hao, M., Fan, G., Zhang, Y., Xin, Y., & Zhang, L. (2019). Preparation and characterization of copper-Brevibacterium cholesterol oxidase hybrid nanoflowers. International Journal of Biological Macromolecules, 126, 539–548.
Cheng, P., Tang, M., Chen, Z., Liu, W., Jiang, X., Pei, X., & Su, W. (2020). Dual-enzyme and NADPH co-embedded organic-inorganic hybrid nanoflowers prepared using biomimetic mineralization for the asymmetric synthesis of (: R)-(-)-pantolactone. Reaction Chemistry and Engineering, 5(10), 1973–1980.
Chen, X., Xu, L., Wang, A., Li, H., Wang, C., Pei, X., … Wu, S. G. (2018). Efficient synthesis of the key chiral alcohol intermediate of Crizotinib using dual-enzyme@CaHPO4 hybrid nanoflowers assembled by mimetic biomineralization.Journal of Chemical Technology and Biotechnology, 94(1), 236–243.
Duan, L., Li, H., & Zhang, Y. (2018). Synthesis of hybrid nanoflower-based carbonic anhydrase for enhanced biocatalytic activity and stability. ACS Omega, 3(12), 18234–18241.
Metelitza, D. I., Litvinchuk, A. V., & Savenkova, M. I. (1991). Peroxidase-catalyzed co-oxidation of halogen-substituted phenols and 4-aminoantipyrine. Journal of Molecular Catalysis, 67(3), 401–411.
Zhong, L., Jiao, X., Hu, H., Shen, X., Zhao, J., Feng, Y., … Jia, S. (2021). Activated magnetic lipase-inorganic hybrid nanoflowers: A highly active and recyclable nanobiocatalyst for biodiesel production. Renewable Energy, 171, 825–832.
Acknowledgements
The authors would also like to thank LCME – UFSC for the technical support during electron microscopy analysis and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. F.P. da Costa: material preparation, data collection, analysis, and original draft. R. O. Henriques: writing, review, and editing. A. Furigo Jr: Project administration.
All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
da Costa, F.P., Henriques, R.O. & Furigo Junior, A. Practical and Rapid Membrane-Based Biosensor for Phenol Using Copper/Calcium-Enzyme Hybrid Nanoflowers. Appl Biochem Biotechnol 195, 86–106 (2023). https://doi.org/10.1007/s12010-022-04101-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-04101-5