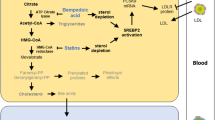
Abstract
The major cause of death worldwide is atherosclerosis-related cardiovascular disease (ACD). Myrtenal was studied to determine control rats were given standard diets and a high-fat diet was given to AS model groups. Atherosclerosis-related cardiovascular disease (ACD) is globally attributed to being a predominant cause of mortality. While the beneficial effects of Myrtenal, the monoterpene from natural compounds, are increasingly being acknowledged, its anti-atherosclerotic activity has not been demonstrated clearly. The present study is proposed to determine the anti-atherosclerotic activity of Myrtenal in high-fat diet-induced atherosclerosis (AS) rat models. Control groups were maintained with standard diets, the AS model rats were provided a high-fat diet, two of the experimental groups fed with a high-fat diet were treated with Myrtenal (50 mg/kg and 100 mg/kg), and one experimental group on high-fat diet was treated with simvastatin (10 mg/kg) for 30 days. The levels of inflammatory cytokines were analyzed using kits. The lipoproteins and the lipid profile were estimated using an auto-analyzer. The atherogenic index and marker enzyme activities were also determined. Serum concentrations of 6-keto-prostaglandin F1α (6-keto-PGF1α), thromboxaneB2 (TXB2), endothelin (ET), and nitric oxide (NO) were measured. The AS model groups indicated altered lipid profile, lipoprotein content, atherogenic index, calcium levels, HMG-CoA reductase activity, collagen level, and mild mineralization indicating atherosclerosis, while the AS-induced Myrtenal-treated groups demonstrated anti-atherogenic activity. The Myrtenal-treated groups exhibited a decreased TC, TG, and LDLc levels; increased HDLc levels; and a decline in the inflammatory cytokines such as CRP, IL-1β, IL-8, and IL-18 when compared to the untreated AS rats. Furthermore, Myrtenal decreased ET, TXB2, and 6-keto-PGF1α levels indicating its anti-atherosclerotic activity. The study results thus indicate that Myrtenal modulates the lipid metabolic pathway to exert its anti-atherosclerotic activity.











Similar content being viewed by others
Availability of Data and Materials
Not applicable.
References
Malekmohammad, K., Bezsonov, E. E., & Rafieian-Kopaei, M. (2021). Role of lipid accumulation and inflammation in atherosclerosis: Focus on molecular and cellular mechanisms. Frontiers in Cardiovascular Medicine, 8, 707529.
Marchio, P., Guerra-Ojeda, S., Vila, J. M., Aldasoro, M., Victor, V. M., & Mauricio, M. D. (2019). Targeting early atherosclerosis: A focus on oxidative stress and inflammation. Oxidative Medicine and Cellular Longevity, 2019, 8563845.
Padro, T., Vilahur, G., Sánchez-Hernández, J., Hernández, M., Antonijoan, R. M., Perez, A., & Badimon, L. (2015). Lipidomic changes of LDL in overweight and moderately hypercholesterolemic subjects taking phytosterol- and omega-3-supplemented milk. Journal of Lipid Research, 56(5), 1043–1056.
Zeng, L., Mathew, A. V., Byun, J., Atkins, K. B., Brosius, F. C., & Pennathur, S. (2018). Myeloperoxidase-derived oxidants damage artery wall proteins in an animal model of chronic kidney disease-accelerated atherosclerosis. Journal of Biological Chemistry, 293(19), 7238–7249.
Gesto, D. S., Pereira, C. M. S., Cerqueira, N. M. F. S., & Sousa, S. F. (2020). An atomic-level perspective of hmg-coa-reductase: The target enzyme to treat hypercholesterolemia. Molecules, 25(17), 3891.
Mohammad, S., Nguyen, H., Nguyen, M., Abdel-Rasoul, M., Nguyen, V., Nguyen, C. D., Nguyen, K. T., Li, L., & Kitzmiller, J. P. (2019). Pleiotropic effects of statins: Untapped potential for statin pharmacotherapy. Current Vascular Pharmacology, 17(3), 239–261.
Taylor, F., Ward, K., Moore, T. H., Burke, M., Davey Smith, G., Casas, J. P., Ebrahim, S. (2013). Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev, 1, CD004816.
Pinal-Fernandez, I., Casal-Dominguez, M., & Mammen, A. L. (2018). Statins: Pros and cons. Medicina Clínica (Barcelona), 150(10), 398–402.
Serban, M. M., Mikhailidis, D. P., Toth, P. P., Grzesiak, M., Mazidi, M., Maciejewski, M., & Banach, M. (2018). The potential role of statins in preeclampsia and dyslipidemia during gestation: A narrative review. Expert Opinion on Investigational Drugs, 27(5), 427–435.
Lokeshkumar, B., Sathishkumar, V., Nandakumar, N., Rengarajan, T., Madankumar, A., & Balasubramanian, M. P. (2015). Anti-Oxidative effect of myrtenal in prevention and treatment of colon cancer induced by 1, 2-Dimethyl Hydrazine (DMH) in experimental animals. Biomol Ther (Seoul), 23(5), 471–478.
Zielinska-Błajet, M., & Feder-Kubis, J. (2020). Monoterpenes and their derivatives—recent development in biological and medical applications. International Journal of Molecular Sciences, 21, 7078.
Corin, K., Baaske, P., Geissler, S., et al. (2011). Structure and function analyses of the purified GPCR human vomeronasal type 1 receptor 1. Science and Reports, 1, 172.
Martins, B. X., Arruda, R. F., Costa, G. A., Jerdy, H., de Souza, S. B., Santos, J. M., de Freitas, W. R., Kanashiro, M. M., de Carvalho, E. C. Q., Sant’Anna, N. F., Antunes, F., Martinez-Zaguilan, R., Okorokova-Facanha, A. L., & Facanha, A. R. (2019). Myrtenal-induced V-ATPase inhibition - A toxicity mechanism behind tumor cell death and suppressed migration and invasion in melanoma. Biochimica et Biophysica Acta - General Subjects, 1863(1), 1–12.
Dragomanova, S., Tancheva, L., Georgieva, M., Georgieva, A., Stoeva, S., Kalfin, R. (2015). Antioxidant mechanism in the preventive effect of myrtenal on Alzheimer’s disease progression on experimental mouse model. European College of Neuropsychopharmacology, Amsterdam, The Nederlands, 2015, Abstract book of ECNP, 25(2), S578–9.
Klisurov, R., Dragomanova, S., Tancheva, L., Kalfin, R. (2017). Study on the neuroprotective mechanisms of myrtenal on experimental rats. 2nd International Biomedical Congress 2017, Sofia, Bulgaria. Abstract book. p. 39.
Kaufmann, D., Dogra, A. K., & Wink, M. (2011). Myrtenal inhibits acetylcholinesterase, a known Alzheimer target. Journal of Pharmacy and Pharmacology, 63(10), 1368–1371.
Corin, K., Baaske, P., Geissler, S., Wienken, C. J., Duhr, S., Braun, D., & Zhang, S. (2011). Structure and function analyses of the purified GPCR human vomeronasal type 1 receptor 1. Science and Reports, 1, 172.
Dragomanova, S., Klisurov, R., Georgieva, M., Lazarova, M., Dishovsky, C., Kalfin, R., et al. (2018). Effect of myrtenal on social behavior and memory of rats. 10th Congress of Toxicology in Developing Countries (CTDC10), 18–21 April, Belgrade, Serbia.
Li, W. X., Qian, P., Guo, Y. T., Gu, L., Jurat, J., Bai, Y., & Zhang, D. F. (2021). Myrtenal and β-caryophyllene oxide screened from Liquidambaris Fructus suppress NLRP3 inflammasome components in rheumatoid arthritis. BMC Complement Med Ther, 21(1), 242.
Ayyasamy, R., & Leelavinothan, P. (2016). Myrtenal alleviates hyperglycaemia, hyperlipidaemia and improves pancreatic insulin level in STZ-induced diabetic rats. Pharmaceutical Biology, 54(11), 2521–2527.
Rathinam, A., & Pari, L. (2016). Myrtenal ameliorates hyperglycemia by enhancing GLUT2 through Akt in the skeletal muscle and liver of diabetic rats. Chemico-Biological Interactions, 256, 161–166.
Papandreou, D., & Hamid, Z. T. (2015). The role of vitamin d in diabetes and cardiovascular disease: An updated review of the literature. Disease Markers, 2015, 580474.
Edwards, C. A., & O’Brien, W. D. (1980). Modified assay for determination of hydroxypro-line in a tissue hydrolyzate. Clinica Chimica Acta, 104, 161–167.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., et al. (1951). Protein measurements with the folinphenol reagent. Journal of Biological Chemistry, 193, 265–275.
Rao, A. V., Ramakrishnan, S., Indirect assessment of hydroxylmethylglutaryl-CoAreductase (NADPH) activity in liver. Clin Chem, 21, 1523–1525.
Itaya, K. (1977). A more sensitive and stable colorimetric determination of free fatty acidsin blood. Journal of Lipid Research, 18, 663–665.
Onat, A., Can, G., Kaya, H., et al. (2010). Atherogenic index of plasma (log10 (triglyceride/high density lipoprotein cholesterol) predicts high blood pressure, diabetes, and vascular events. Journal of Clinical Lipidology, 4, 89–98.
Miura, Y., & Suzuki, H. (2019). Dyslipidemia and atherosclerotic carotid artery stenosis. Vessel Plus, 3, 1.
Shrivastava, A., Chaturvedi, U., Singh, S. V., Saxena, J. K., & Bhatia, G. (2013). Lipid lowering and antioxidant effect of miglitol in triton treated hyperlipidemic and high fat diet induced obese rats. Lipids, 48(6), 597–607.
Gianazza, E., Brioschi, M., Fernandez, A. M., & Banfi, C. (2019). Lipoxidation in cardiovascular diseases. Redox Biol, 23, 101119.
Khatana, C., Saini, N. K., Chakrabarti, S., Saini, V., Sharma, A., Saini, R. V., Saini, A. K. (2020). Mechanistic insights into the oxidized low-density lipoprotein-induced atherosclerosis. Oxidative Medicine and Cellular Longevity, 2020.
Hoenig, M. R. (2008). Implications of the obesity epidemic for lipid-lowering therapy: Non-HDL cholesterol should replace LDL cholesterol as the primary therapeutic target. Vasc Health Risk Manag, 4(1), 143–156.
Xiao, C. W., Wood, C. M., Swist, E., Nagasaka, R., Sarafin, K., Gagnon, C., Fernandez, L., Faucher, S., Wu, H. X., Kenney, L., & Ratnayake, W. M. (2016). Cardio-metabolic disease risks and their associations with circulating 25-hydroxyvitamin D and omega-3 levels in South Asian and White Canadians. PLoS ONE, 11(1), e0147648.
Zaric, B. L., Radovanovic, J. N., Gluvic, Z., Stewart, A. J., Essack, M., Motwalli, O., Gojobori, T., & Isenovic, E. R. (2020). Atherosclerosis linked to aberrant amino acid metabolism and immunosuppressive amino acid catabolizing enzymes. Frontiers in Immunology, 11, 551758.
Subramani, C., Rajakkannu, A., Rathinam, A., Gaidhani, S., Raju, I., Kartar Singh, D. V. (2017). Anti-atherosclerotic activity of root bark of Premna integrifolia Linn. in high fat diet induced atherosclerosis model rats. J Pharm Anal, 7(2), 123–128.
Abdelhalim, M. A., Siiddiqi, N. J., Alhomida, A. S., & Al-Ayed, M. S. (2008). Effects of feeding periods of high cholesterol and saturated fat diet on blood biochemistry and hydroxyproline fractions in rabbits. Bioinformatics and Biology Insights, 2, 95–100.
Kontush, A., Lhomme, M., & Chapman, M. J. (2013). Unraveling the complexities of the HDL lipidome. Journal of Lipid Research, 54(11), 2950–2963.
Misra, B. B., Puppala, S. R., Comuzzie, A. G., Mahaney, M. C., VandeBerg, J. L., Olivier, M., & Cox, L. A. (2019). Analysis of serum changes in response to a high-fat high cholesterol diet challenge reveals metabolic biomarkers of atherosclerosis. PLoS ONE, 14(4), e0214487.
Wu, Y., Pan, N., An, Y., Xu, M., Tan, L., & Zhang, L. (2021). Diagnostic and prognostic biomarkers for myocardial infarction. Front Cardiovasc Med, 7, 617277.
Parsanathan, R., & Jain, S. K. (2019). Novel invasive and noninvasive cardiac-specific biomarkers in obesity and cardiovascular diseases. Metabolic Syndrome and Related Disorders, 18(1), 10–30.
Hesari, M., Mohammadi, P., Khademi, F., Shackebaei, D., Momtaz, S., Moasefi, N., Farzaei, M. H., & Abdollahi, M. (2021). Current advances in the use of nanophytomedicine therapies for human cardiovascular diseases. International Journal of Nanomedicine, 16, 3293–3315.
Burnstock, G., & Pelleg, A. (2015). Cardiac purinergic signalling in health and disease. Purinergic Signal, 11(1), 1–46.
Tan, B. L., & Norhaizan, M. E. (2019). Effect of high-fat diets on oxidative stress, cellular inflammatory response and cognitive function. Nutrients, 11(11), 2579.
Li, B., Xia, Y., & Hu, B. (2020). Infection and atherosclerosis: TLR-dependent pathways. Cellular and Molecular Life Sciences, 77(14), 2751–2769.
Schumacher, M. M., Jun, D. J., Johnson, B. M., & DeBose-Boyd, R. A. (2018). UbiA prenyltransferase domain-containing protein-1 modulates HMG-CoA reductase degradation to coordinate synthesis of sterol and nonsterol isoprenoids. Journal of Biological Chemistry, 293(1), 312–323.
Smith, L. R., & Barton, E. R. (2014). Collagen content does not alter the passive mechanical properties of fibrotic skeletal muscle in mdx mice. American Journal of Physiology. Cell Physiology, 306(10), C889–C898.
Saigusa, R., Winkels, H., & Ley, K. (2020). T cell subsets and functions in atherosclerosis. Nature Reviews. Cardiology, 17(7), 387–401.
Bäck, M., Yurdagul, A., Tabas, I., Öörni, K., & Kovanen, P. T. (2019). Inflammation and its resolution in atherosclerosis: Mediators and therapeutic opportunities. Nature Reviews. Cardiology, 16(7), 389–406.
Cheng, Z., Jia, W., Tian, X., Jiang, P., Zhang, Y., Li, J., Tian, C., Liu, J. (2020). Cotinine inhibits TLR4/NF-κB signaling pathway and improves deep vein thrombosis in rats. Biosci Rep, 40(6), BSR20201293.
Author information
Authors and Affiliations
Contributions
Liyan Yu, Hongguang Liu, Xiaoxia Ma, Vidya Devanathadesikan Seshadri, and Xuan Gao contributed equally.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
All authors have their consent to participate.
Consent to Publish
All authors have their consent to publish their work.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yu, L., Liu, H., Ma, X. et al. Anti-atherosclerotic Effects of Myrtenal in High-Fat Diet-Induced Atherosclerosis in Rats. Appl Biochem Biotechnol 194, 5717–5733 (2022). https://doi.org/10.1007/s12010-022-04044-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-04044-x