Abstract
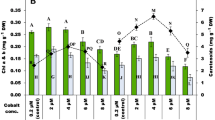
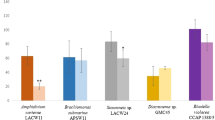
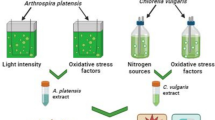
There has been increasing demands worldwide for bioactive compounds of natural origins, especially for the nutraceutical and food-supplement sectors. In this context, microalgae are viewed as sustainable sources of molecules with an array of health benefits. For instance, astaxanthin is a xanthophyll pigment with powerful antioxidant capacity produced by microalgae such as the chlorophyte Haematococcus sp., which is regarded as the most suitable organism for the mass production of this pigment. In this study, three Haematococcus sp. strains were cultivated using a batch mode under favourable conditions to promote vegetative growth. Their environment was altered in a second phase using a higher and constant illumination regime combined with either exposure to blue LED light, an osmotic shock (with NaCl addition) or supplementation with a phytohormone (gibberellic acid, GA3), a plant extract (ginger), an herbicide (molinate) or an oxidant reagent (hydrogen peroxide). The effects of these stressors were evaluated in terms of antioxidant response and astaxanthin and β-carotene accumulation. Overall, strain CCAP 34/7 returned the highest Trolox Equivalent Antioxidant Capacity (TEAC) response (14.1–49.1 µmoL Trolox eq. g− 1 of DW), while the highest antioxidant response with the Folin–Ciocalteu (FC) was obtained for strain RPFW01 (62.5–155 µmoL Trolox eq. g− 1 of DW). The highest β-β-carotene content was found in strain LAFW15 when supplemented with the ginger extract (4.8 mg. g− 1). Strain RPFW01 exposed to blue light returned the highest astaxanthin yield (2.8 mg. g− 1), 5-fold that of strain CCAP 34/7 on average. This study documents the importance of screening several strains when prospecting for species with potential to produce high-value metabolites. It highlights that strain-specific responses can ensue from exposure of cells to a variety of stressors, which is important for the adequate tailoring of a biorefinery pipeline.
Graphical Abstract










Similar content being viewed by others
Data Availability
Data will be made available on request.
References
Apel, K., & Hirt, H. (2004). Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology, 55, 373–399. https://doi.org/10.1146/annurev.arplant.55.031903.141701
Uttara, B., Singh, A. V., Zamboni, P., & Mahajan, R. T. (2009). Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Current Neuropharmacology, 7(1), 65–74. https://doi.org/10.2174/157015909787602823
Gilgun-Sherki, Y., Melamed, E., & Offen, D. (2001). Oxidative stress induced-neurodegenerative diseases: The need for antioxidants that penetrate the blood brain barrier. Neuropharmacology, 40(8), 959–975. https://doi.org/10.1016/s0028-3908(01)00019-3
Nunomura, A., Castellani, R. J., Zhu, X., Moreira, P. I., Perry, G., & Smith, M. A. (2006). Involvement of oxidative stress in Alzheimer disease. Journal of Neuropathology and Experimental Neurology, 65(7), 631–641. https://doi.org/10.1097/01.jnen.0000228136.58062.bf
Guedes, A. C., Amaro, H. M., & Malcata, F. X. (2011). Microalgae as sources of high added-value compounds–A brief review of recent work. Biotechnology Progress, 27(3), 597–613. https://doi.org/10.1002/btpr.575
Simopoulos, A. P. (2002). The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomedicine & Pharmacotherapy, 56(8), 365–379. https://doi.org/10.1016/s0753-3322(02)00253-6
Barkia, I., Saari, N., & Manning, S. R. (2019). Microalgae for high-value products towards human health and nutrition. Marine Drugs, 17(5), 304. Published 2019 May 24. https://doi.org/10.3390/md17050304
Aruoma, O. I. (1998). Free radicals, oxidative stress, and antioxidants in human health and disease. Journal of the American Oil Chemists' Society, 75(2), 199–212. https://doi.org/10.1007/s11746-998-0032-9
Goiris, K., Muylaert, K., Fraeye, I., Foubert, I., De Brabanter, J., & De Cooman, L. (2012). Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. Journal of Applied Phycology, 24(6), 1477–1486. https://doi.org/10.1007/s10811-012-9804-6
Cepoi, L. (2014). Antioxidant activity in Haematococcus Pluvialis cells during the vital cycle. Microbial Biotechnology. Ediția 2, 9–10, pp 25–29. ISBN 978-9975-4432-8-9
Fiedor, J., & Burda, K. (2014). Potential role of carotenoids as antioxidants in human health and disease. Nutrients, 6(2), 466–488. Published 2014 Jan 27. https://doi.org/10.3390/nu6020466
Ambati, R. R., Phang, S. M., Ravi, S., & Aswathanarayana, R. G. (2014). Astaxanthin: sources, extraction, stability, biological activities and its commercial applications–A review. Marine Drugs, 12(1), 128–152. Published 2014 Jan 7. https://doi.org/10.3390/md12010128
Gong, M., & Bassi, A. (2016). Carotenoids from microalgae: A review of recent developments. Biotechnology Advances, 34(8), 1396–1412. https://doi.org/10.1016/j.biotechadv.2016.10.005
Li, X., Wang, X., Duan, C., et al. (2020). Biotechnological production of astaxanthin from the microalga Haematococcus pluvialis. Biotechnology Advances, 43, 107602. https://doi.org/10.1016/j.biotechadv.2020.107602
Davinelli, S., Nielsen, M. E., & Scapagnini, G. (2018). Astaxanthin in skin health, repair, and disease: A comprehensive review. Nutrients, 10(4), 522. Published 2018 Apr 22. https://doi.org/10.3390/nu10040522
Khoo, K. S., Lee, S. Y., Ooi, C. W., et al. (2019). Recent advances in biorefinery of astaxanthin from Haematococcus pluvialis. Bioresource Technology, 288, 121606. https://doi.org/10.1016/j.biortech.2019.121606
Stachowiak, B., & Szulc, P. (2021). Astaxanthin for the Food Industry. Molecules, 26(9), 2666. Published 2021 May 2. https://doi.org/10.3390/molecules26092666
Dong, S., Huang, Y., Zhang, R., Wang, S., & Liu, Y. (2014). Four different methods comparison for extraction of astaxanthin from green alga haematococcus pluvialis. The Scientific World Journal, 2014. https://doi.org/10.1155/2014/694305
Saw, C. L., Yang, A. Y., Guo, Y., & Kong, A. N. (2013). Astaxanthin and omega-3 fatty acids individually and in combination protect against oxidative stress via the Nrf2-ARE pathway. Food and Chemical Toxicology, 62, 869–875. https://doi.org/10.1016/j.fct.2013.10.023
Fassett, R. G., & Coombes, J. S. (2012). Astaxanthin in cardiovascular health and disease. Molecules, 17(2), 2030–2048. https://doi.org/10.3390/molecules17022030 Published 2012 Feb 20
Giannaccare, G., Pellegrini, M., Senni, C., Bernabei, F., Scorcia, V., & Cicero, A. F. G. (2020). Clinical applications of astaxanthin in the treatment of ocular diseases: emerging insights. Marine Drugs, 18(5), 239. Published 2020 May 1. https://doi.org/10.3390/md18050239
Donoso, A., González-Durán, J., Muñoz, A. A., González, P. A., & Agurto-Muñoz, C. (2021). "Therapeutic uses of natural astaxanthin: An evidence-based review focused on human clinical trials" Pharmacological Research, 166, 105479. https://doi.org/10.1016/j.phrs.2021.105479
Mularczyk, M., Michalak, I., & Marycz, K. (2020). Astaxanthin and other nutrients from Haematococcus pluvialis-Multifunctional applications. Marine Drugs, 18(9), 459. Published 2020 Sep 7. https://doi.org/10.3390/md18090459
Panis, G., & Carreon, J. R. (2016). Commercial astaxanthin production derived by green alga Haematococcus pluvialis: A microalgae process model and a techno-economic assessment all through production line. Algal Research, 18, 175–190. https://doi.org/10.1016/j.algal.2016.06.007
Guerin, M., Huntley, M. E., & Olaizola, M. (2003). Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol, 21(5), 210–216. https://doi.org/10.1016/S0167-7799(03)00078-7
Lorenz, R. T., & Cysewski, G. R. (2000). Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends Biotechnol, 18(4), 160–167. https://doi.org/10.1016/s0167-7799(00)01433-5
ReportLinker. (2020). Astaxanthin market by source, form, method of production, application and region - Global forecast to 2022. Accessed 5 Nov. https://www.reportlinker.com/p05226982/Astaxanthin-Market-by-Source-Form-Method-of-Production-Application-And-Region-Global-Forecast-to.html
Insights, G. M. (2020). Astaxanthin Market size to exceed $880mn by 2026. Published 2019. Accessed 5 Nov. https://www.gminsights.com/pressrelease/astaxanthin-market.
Pérez-López, P., González-García, S., Jeffryes, C. S., Agathos, S. N., McHugh, E. L., Walsh, D. J., & Moreira, M. T. (2014). Life cycle assessment of the production of the red antioxidant carotenoid astaxanthin by microalgae: From lab to pilot scale. Journal of Cleaner Production, 64, 332–344. https://doi.org/10.1016/j.jclepro.2013.07.011
Li, J., Zhu, D., Niu, J., Shen, S., & Wang, G. (2011). An economic assessment of astaxanthin production by large scale cultivation of Haematococcus pluvialis. Biotechnology Advances, 29(6), 568–574. https://doi.org/10.1016/j.biotechadv.2011.04.001
Higuera-Ciapara, I., Félix-Valenzuela, L., & Goycoolea, F. M. (2006). Astaxanthin: A review of its chemistry and applications. Critical Reviews in Food Science and Nutrition, 46(2), 185–196. https://doi.org/10.1080/10408690590957188
Saeed, M. U., Hussain, N., Shahbaz, A., Hameed, T., Iqbal, H. M. N., & Bilal, M. (2021). Bioprospecting microalgae and cyanobacteria for biopharmaceutical applications. Journal of Basic Microbiology. https://doi.org/10.1002/JOBM.202100445
Kumar Patel, A., Pralhad Vadrale, A., Tseng, Y. S., Chen, C. W., Dong, C. D., & Rani Singhania, R. (2022). Bioprospecting of marine microalgae from Kaohsiung Seacoast for lutein and lipid production. Bioresource Technology, 126928. https://doi.org/10.1016/j.biortech.2022.126928
Mc Gee, D., Archer, L., Smyth, T. J., Fleming, G. T. A., & Touzet, N. (2020). Bioprospecting and LED-based spectral enhancement of antimicrobial activity of microalgae isolated from the west of Ireland. Algal Research, 45, 101704. https://doi.org/10.1016/j.algal.2019.101704
Boussiba, S., Bing, W., Yuan, J. P., Zarka, A., & Chen, F. (1999). Changes in pigments profile in the green alga Haeamtococcus pluvialis exposed to environmental stresses. Biotechnology Letters, 21(7), 601–604. https://doi.org/10.1023/A:1005507514694
Göksan, T., & Ak, I. (2006). Vegetative growth of the green alga Haematococcus pluvialis cultivated in different light-path lengths. Asian Journal of Plant Sciences, 5(3), 455–460. https://doi.org/10.3923/ajps.2006.455.460
Ma, R. Y. N., & Chen, F. (2001). Enhanced production of free trans-astaxanthin by oxidative stress in the cultures of the green microalga Chlorococcum sp. Process Biochemistry (Barking, UK), 36(12), 1175–1179. https://doi.org/10.1016/S0032-9592(01)00157-1
Orosa, M., Valero, J. F., Herrero, C., & Abalde, J. (2001). Comparison of the accumulation of astaxanthin in Haematococcus pluvialis and other green microalgae under N-starvation and high light conditions. Biotechnology Letters, 23(13), 1079–1085. https://doi.org/10.1023/A:1010510508384
Lababpour, A., Hada, K., Shimahara, K., Katsuda, T., & Katoh, S. (2004). Effects of nutrient supply methods and illumination with blue light emitting diodes (LEDs) on astaxanthin production by Haematococcus pluvialis. Journal of Bioscience and Bioengineering, 98(6), 452–456. https://doi.org/10.1016/S1389-1723(05)00311-7
Haque, F., Dutta, A., Thimmanagari, M., & Chiang, Y. W. (2016). Intensified green production of astaxanthin from Haematococcus pluvialis. Food and Bioproducts Processing, 99, 1–11. https://doi.org/10.1016/j.fbp.2016.03.002
Torres-Carvajal, L. K., Gonzalez-Delgado, A. D., Barajas-Solano, A. F., Suarez-Gelvez, J. H., & Urbina-Suarez, N. A. (2017). Astaxanthin production from Haematococcus pluvialis: Effects of light wavelength and salinity. Contemporary Engineering Sciences, 10(35), 1739–1746. https://doi.org/10.12988/ces.2017.711196
Hunt, R. W., Chinnasamy, S., Bhatnagar, A., & Das, K. C. (2010). Effect of biochemical stimulants on biomass productivity and metabolite content of the microalga, Chlorella sorokiniana. Applied Biochemistry and Biotechnology, 162(8), 2400–2414. https://doi.org/10.1007/s12010-010-9012-2
Fábregas, J., Domínguez, A., Regueiro, M., Maseda, A., & Otero, A. (2000). Optimization of culture medium for the continuous cultivation of the microalga Haematococcus pluvialis. Applied Microbiology and Biotechnology, 53(5), 530–535. https://doi.org/10.1007/s002530051652
Parkes, R., Archer, L., Gee, D. M., Smyth, T. J., Gillespie, E., & Touzet, N. (2021). Differential responses in EPA and fucoxanthin production by the marine diatom Stauroneis sp. under varying cultivation conditions. Biotechnology Progress, 37(6), e3197. https://doi.org/10.1002/btpr.3197
McGee, D., Archer, L., Fleming, G. T. A., Gillespie, E., & Touzet, N. (2020). Influence of spectral intensity and quality of LED lighting on photoacclimation, carbon allocation and high-value pigments in microalgae. Photosynthesis Research, 143(1), 67–80. https://doi.org/10.1007/s11120-019-00686-x
Kumar, S., Stecher, G., & Tamura, K. (2016). MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33(7), 1870–1874. https://doi.org/10.1093/molbev/msw054
Thompson, J. D., Higgins, D. G., & Gibson, T. J. (1994). CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research, 22(22), 4673–4680. https://doi.org/10.1093/nar/22.22.4673
Tamura, K., Stecher, G., Peterson, D., Filipski, A., & Kumar, S. (2013). MEGA6: Molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30(12), 2725–2729. https://doi.org/10.1093/molbev/mst197
Garvey, M., Moriceau, B., & Passow, U. (2007). Applicability of the FDA assay to determine the viability of marine phytoplankton under different environmental conditions. Marine Ecology Progress Series, 352, 17–26. https://doi.org/10.3354/meps07134
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology & Medicine, 26(9–10), 1231–1237. https://doi.org/10.1016/s0891-5849(98)00315-3
Archer, L., McGee, D., Parkes, R., et al. (2021). Antioxidant bioprospecting in microalgae: Characterisation of the potential of two marine heterokonts from Irish waters. Applied Biochemistry and Biotechnology, 193(4), 981–997. https://doi.org/10.1007/s12010-020-03467-8
Wang, S., Meng, Y., Liu, J., Cao, X., & Xue, S. (2018). Accurate quantification of astaxanthin from Haematococcus pluvialis using DMSO extraction and lipase-catalyzed hydrolysis pretreatment. Algal Research, 35, 427–431. https://doi.org/10.1016/j.algal.2018.08.029
Mc Gee, D., Archer, L., Paskuliakova, A., et al. (2018). Rapid chemotaxonomic profiling for the identification of high-value carotenoids in microalgae. Journal of Applied Phycology, 30(1), 385–399. https://doi.org/10.1007/s10811-017-1247-7
Kobayashi, M., Kurimura, Y., & Tsuji, Y. (1997). Light-independent, astaxanthin production by the green microalga Haematococcus pluvialis under salt stress. Biotechnology Letters, 19(6), 507–509. https://doi.org/10.1023/A:1018372900649
Herrero, M., Sánchez-Camargo, A., del Cifuentes, P., & Ibáñez, A. (2015). Plants, seaweeds, microalgae and food by-products as natural sources of functional ingredients obtained using pressurized liquid extraction and supercritical fluid extraction. TrAC Trends in Analytical Chemistry, 71, 26–38. https://doi.org/10.1016/j.trac.2015.01.018
Mascia, F., Girolomoni, L., Alcocer, M. J. P., et al. (2017). Functional analysis of photosynthetic pigment binding complexes in the green alga Haematococcus pluvialis reveals distribution of astaxanthin in Photosystems. Scientific Reports, 7(1). https://doi.org/10.1038/s41598-017-16641-6
Aburai, N., Sumida, D., & Abe, K. (2015). Effect of light level and salinity on the composition and accumulation of free and ester-type carotenoids in the aerial microalga Scenedesmus sp. (Chlorophyceae). Algal Research, 8, 30–36. https://doi.org/10.1016/j.algal.2015.01.005
Suyono, E. A., Aminin, Pradani, L., et al. (2015). Combination of blue, red, white, and ultraviolet lights for increasing carotenoids and biomass of microalga Haematococcus pluvialis. Procedia Environmental Sciences, 28, 399–405. https://doi.org/10.1016/j.proenv.2015.07.049
Gómez, P. I., Haro, P., Lagos, P., et al. (2016). Intraspecific variability among Chilean strains of the astaxanthin-producing microalga Haematococcus pluvialis (Chlorophyta): An opportunity for its genetic improvement by simple selection. Journal of Applied Phycology, 28(4), 2115–2122. https://doi.org/10.1007/S10811-015-0777-0
Mostafa, N., Omar, H., Tan, S. G., & Napis, S. (2011). Studies on the genetic variation of the green unicellular alga Haematococcus pluvialis (Chlorophyceae) obtained from different geographical locations using ISSR and RAPD molecular marker. Molecules, 16(3), 2599–2608. https://doi.org/10.3390/MOLECULES16032599
Giannelli, L., Yamada, H., Katsuda, T., & Yamaji, H. (2015). Effects of temperature on the astaxanthin productivity and light harvesting characteristics of the green alga Haematococcus pluvialis. Journal of Bioscience and Bioengineering, 119(3), 345–350. https://doi.org/10.1016/j.jbiosc.2014.09.002
Karuppan, R., Javee, A., Gopidas, S. K., & Subramani, N. (2019). Influence of agriculture fertilizer for the enhanced growth and astaxanthin production from Haematococcus lacustris RRGK isolated from Himachal Pradesh, India. SN Applied Sciences, 1(6). https://doi.org/10.1007/S42452-019-0543-Z
Allewaert, C. C., Vanormelingen, P., Daveloose, I., Verstraete, T., & Vyverman, W. (2017). Intraspecific trait variation affecting astaxanthin productivity in two Haematococcus (Chlorophyceae) species. Algal Research, 21, 191–202. https://doi.org/10.1016/j.algal.2016.10.021
Droop, M. R. (1955). Some factors governing encystment in Haematococcus pluvialis. Archiv für Mikrobiologie, 21(3), 267–272. https://doi.org/10.1007/BF00412349
Allewaert, C. C., Vanormelingen, P., Pröschold, T., et al. (2019). Species diversity in European Haematococcus pluvialis (Chlorophyceae, Volvocales). Phycologia, 54(6), 583–598. https://doi.org/10.2216/15-55.1
Noroozi, M., Omar, H., Napis, S., Hejazi, M. A., & Science, F. (2012). Comparative biodiversity and effect of different media on growth and astaxanthin content of nine geographical strains of Haematococcus pluvialis. African Journal of Biotechnology, 11(84), 15049–15059. https://doi.org/10.4314/ajb.v11i84
Chekanov, K., Schastnaya, E., Solovchenko, A., & Lobakova, E. (2017). Effects of CO2enrichment on primary photochemistry, growth and astaxanthin accumulation in the chlorophyte Haematococcus pluvialis. The Journal of Photochemistry and Photobiology B: Biology, 171, 58–66. https://doi.org/10.1016/j.jphotobiol.2017.04.028
Zhang, B. Y., Geng, Y. H., Li, Z. K., Hu, H. J., & Li, Y. G. (2009). Production of astaxanthin from Haematococcus in open pond by two-stage growth one-step process. Aquaculture, 295(3–4), 275–281. https://doi.org/10.1016/j.aquaculture.2009.06.043
Gao, Z., Meng, C., Chen, Y. C., et al. (2015). Comparison of astaxanthin accumulation and biosynthesis gene expression of three Haematococcus pluvialis strains upon salinity stress. Journal of Applied Phycology, 27(5), 1853–1860. https://doi.org/10.1007/s10811-014-0491-3
Coutinho Rodrigues, O. H., Itokazu, A. G., Rörig, L., et al. (2021). Evaluation of astaxanthin biosynthesis by Haematococcus pluvialis grown in culture medium added of cassava wastewater. International Biodeterioration and Biodegradation, 163, 105269. https://doi.org/10.1016/J.IBIOD.2021.105269
Azizi, M., Moteshafi, H., & Hashemi, M. (2020). Distinctive nutrient designs using statistical approach coupled with light feeding strategy to improve the Haematococcus pluvialis growth performance and astaxanthin accumulation. Bioresource Technology, 300, 122594. https://doi.org/10.1016/j.biortech.2019.122594
Harker, M., Tsavalos, A. J., & Young, A. J. (1995). Use of response surface methodology to optimise carotenogenesis in the microalga, Haematococcus pluvialis. Journal of Applied Phycology, 7(4), 399–406. https://doi.org/10.1007/BF00003797
Han, D., Wang, J., Sommerfeld, M., & Hu, Q. (2012). Susceptibility and protective mechanisms of motile and non motile cells of haematococcus pluvialis (chlorophyceae) to photooxidative stress. Journal of Phycology, 48(3), 693–705. https://doi.org/10.1111/J.1529-8817.2012.01147.X
Kobayashi, M., Kurimura, Y., Kakizono, T., Nishio, N., & Tsuji, Y. (1997). Morphological changes in the life cycle of the green alga Haematococcus pluvialis. Journal of Fermentation and Bioengineering, 84(1), 94–97. https://doi.org/10.1016/S0922-338X(97)82794-8
Prior, R. L., Wu, X., & Schaich, K. (2005). Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. Journal of Agricultural and Food Chemistry, 53(10), 4290–4302. https://doi.org/10.1021/jf0502698
Goiris, K., Colen, W., Van, Wilches, I., León-Tamariz, F., De Cooman, L., & Muylaert, K. (2015). Impact of nutrient stress on antioxidant production in three species of microalgae. ALGAL, 7, 51–57. https://doi.org/10.1016/j.algal.2014.12.002
Arts, M. J. T., Haenen, G. R. M., Voss, H. P., & Bast, A. (2004). Antioxidant capacity of reaction products limits the applicability of the Trolox Equivalent Antioxidant Capacity (TEAC) assay. Food and Chemical Toxicology, 42(1), 45–49. https://doi.org/10.1016/j.fct.2003.08.004
Choochote, W., Suklampoo, L., & Ochaikul, D. (2014). Evaluation of antioxidant capacities of green microalgae. Journal of Applied Phycology, 26(1), 43–48. https://doi.org/10.1007/s10811-013-0084-6
Morowvat, M. H., & Ghasemi, Y. (2016). Evaluation of antioxidant properties of some naturally isolated microalgae: Identification and characterization of the most efficient strain. Biocatalysis and Agricultural Biotechnology, 8, 263–269. https://doi.org/10.1016/j.bcab.2016.09.010
Li, H. B., Cheng, K. W., Wong, C. C., Fan, K. W., Chen, F., & Jiang, Y. (2007). Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chemistry, 102(3), 771–776. https://doi.org/10.1016/j.foodchem.2006.06.022
Banskota, A. H., Stefanova, R., Sperker, S., Lall, S., Craigie, J. S., & Hafting, J. T. (2014). Lipids isolated from the cultivated red alga Chondrus crispus inhibit nitric oxide production. Journal of Applied Phycology, 26(3), 1565–1571. https://doi.org/10.1007/s10811-013-0174-5
Assunção, M. F. G., Amaral, R., Martins, C. B., et al. (2016). Screening microalgae as potential sources of antioxidants. Journal of Applied Phycology, 29(2), 865–877. https://doi.org/10.1007/S10811-016-0980-7
Barone, M. E., Parkes, R., Herbert, H., et al. (2021). Comparative response of marine microalgae to H2O2-induced oxidative stress. Applied Biochemistry and Biotechnology, 193(12), 4052–4067. https://doi.org/10.1007/s12010-021-03690-x
Soong, Y. Y., & Barlow, P. J. (2004). Antioxidant activity and phenolic content of selected fruit seeds. Food Chemistry, 88(3), 411–417. https://doi.org/10.1016/J.FOODCHEM.2004.02.003
Wong, C. C., Li, H., Bin, Cheng, K. W., & Chen, F. (2006). A systematic survey of antioxidant activity of 30 Chinese medicinal plants using the ferric reducing antioxidant power assay. Food Chemistry, 97(4), 705–711. https://doi.org/10.1016/J.FOODCHEM.2005.05.049
Piotrowska-Niczyporuk, A., & Bajguz, A. (2014). The effect of natural and synthetic auxins on the growth, metabolite content and antioxidant response of green alga Chlorella vulgaris (Trebouxiophyceae). Plant Growth Regulation, 73(1), 57–66. https://doi.org/10.1007/s10725-013-9867-7
Cirulis, J. T., Scott, J. A., & Ross, G. M. (2013). Management of oxidative stress by microalgae. Canadian Journal of Physiology and Pharmacology, 91(1), 15–21. https://doi.org/10.1139/cjpp-2012-0249
Coulombier, N., Jauffrais, T., & Lebouvier, N. (2021). Marine drugs antioxidant compounds from microalgae: A review. Marine Drugs, 19(10), 549. https://doi.org/10.3390/md19100549
Tamaki, S., Mochida, K., & Suzuki, K. (2021). Diverse biosynthetic pathways and protective functions against environmental stress of antioxidants in microalgae. Plants (Basel), 10(6), 1250. Published 2021 Jun 19. https://doi.org/10.3390/plants10061250
Coulombier, N., Nicolau, E., Le Déan, L., Antheaume, C., Jauffrais, T., & Lebouvier, N. (2020). Impact of light intensity on antioxidant activity of tropical microalgae. Marine Drugs, 18(2), 122. https://doi.org/10.3390/MD18020122
Coulombier, N., Nicolau, E., Le Déan, L., et al. (2020). Effects of nitrogen availability on the antioxidant activity and carotenoid content of the microalgae Nephroselmis sp. Marine Drugs, 18(9), 453. https://doi.org/10.3390/MD18090453
Maadane, A., Merghoub, N., Ainane, T., et al. (2015). Antioxidant activity of some Moroccan marine microalgae: Pufa profiles, carotenoids and phenolic content. Journal of Biotechnology, 215, 13–19. https://doi.org/10.1016/j.jbiotec.2015.06.400
Kobayashi, M., & Sakamoto, Y. (1999). Singlet oxygen quenching ability of astaxanthin esters from the green alga Haematococcus pluvialis. Biotechnology Letters, 21(4), 265–269. https://doi.org/10.1023/A:1005445927433
Wan, M., Zhang, J., Hou, D., et al. (2014). The effect of temperature on cell growth and astaxanthin accumulation of Haematococcus pluvialis during a light-dark cyclic cultivation. Bioresource Technology, 167, 276–283. https://doi.org/10.1016/j.biortech.2014.06.030
Grewe, C., & Griehl, C. (2008). Time- and media-dependent secondary carotenoid accumulation in Haematococcus pluvialis. Biotechnology Journal, 3(9–10), 1232–1244. https://doi.org/10.1002/BIOT.200800067
Orosa, M., Franqueira, D., Cid, A., & Abalde, J. (2001). Carotenoid accumulation in Haematococcus pluvialis in mixotrophic growth. Biotechnology Letters, 23(5), 373–378. https://doi.org/10.1023/A:1005624005229
Casella, P., Iovine, A., Mehariya, S., Marino, T., Musmarra, D., & Molino, A. (2020). Smart method for carotenoids characterization in Haematococcus pluvialis red phase and evaluation of astaxanthin thermal stability. Antioxidants (Basel, Switzerland), 9(5). https://doi.org/10.3390/antiox9050422
Wang, B., Zarka, A., Trebst, A., & Boussiba, S. (2003). Astaxanthin accumulation in Haematococcus pluvialis (Chlorophyceae) as an active photoprotective process under high irradiance. Journal of Phycology, 39(6), 1116–1124. https://doi.org/10.1111/j.0022-3646.2003.03-043.x
Lv, H., Xia, F., Liu, M., Cui, X., Wahid, F., & Jia, S. (2016). Metabolomic profiling of the astaxanthin accumulation process induced by high light in Haematococcus pluvialis. Algal Research, 20, 35–43. https://doi.org/10.1016/j.algal.2016.09.019
Guo, H., Li, T., Zhao, Y., & Yu, X. (2021). Role of copper in the enhancement of astaxanthin and lipid coaccumulation in Haematococcus pluvialis exposed to abiotic stress conditions. Bioresource Technology, 335, 125265. https://doi.org/10.1016/J.BIORTECH.2021.125265
Ma, R., Thomas-Hall, S. R., Chua, E. T., et al. (2018). Blue light enhances astaxanthin biosynthesis metabolism and extraction efficiency in Haematococcus pluvialis by inducing haematocyst germination. Algal Research, 35, 215–222. https://doi.org/10.1016/j.algal.2018.08.023
Katsuda, T., Shimahara, K., Shiraishi, H., Yamagami, K., Ranjbar, R., & Katoh, S. (2006). Effect of flashing light from blue light emitting diodes on cell growth and astaxanthin production of Haematococcus pluvialis. Journal of Bioscience and Bioengineering, 102(5), 442–446. https://doi.org/10.1263/jbb.102.442
Ma, R., Thomas-Hall, S. R., Chua, E. T., et al. (2018). Gene expression profiling of astaxanthin and fatty acid pathways in Haematococcus pluvialis in response to different LED lighting conditions. Bioresource Technology, 250, 591–602. https://doi.org/10.1016/j.biortech.2017.11.094
Martín, J. F., Gudiña, E., & Barredo, J. L. (2008). Conversion of beta-carotene into astaxanthin: Two separate enzymes or a bifunctional hydroxylase-ketolase protein? Microbial Cell Factories, 7, 3. Published 2008 Feb 20. https://doi.org/10.1186/1475-2859-7-3
Acknowledgements
The authors would like to thank the technical staff of the School of Science at the Institute of Technology Sligo, specifically JohnJoe Mc Gloin and Mary Connolly for their support.
Funding
This research was financially supported by the IT Sligo President’s Bursary Fund and the VES4US project funded by the European Union’s Horizon 2020 Research and Innovation Programme under grant agreement no 801338.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study methodology, writing review, and editing. Conceptualisation, investigation, formal analysis, data curation, visualisation, project administration and analysis were performed by Rachel Parkes, while supervised by Nicolas Touzet who was involved in conceptualisation, resources, funding acquisition and validation. The first draft of the manuscript was written by Rachel Parkes and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
No ethical approval is required for this study.
Consent to Participate
This study did not require approval in this section.
Consent to Publish
This study did not require approval in this section.
Declarations of Author’s Agreement to Authorship and Submission
All authors have agreed to authorship and the submission of this manuscript for peer review and publication in Applied Biochemistry and Biotechnology.
Conflict of Interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Three Haematococcus sp. strains were exposed to varying stressors via a two-stage cultivation.
• The Irish strain RPFW01 returned the highest antioxidant response with the FC assay (62.5–155 µmoL Trolox eq. g− 1 of DW).
• The CCAP 34/7 strain returned the highest antioxidant response with the TEAC assay (14.1–49.1 µmoL Trolox eq. g− 1 of DW).
• The highest astaxanthin (2.8 mg. g− 1) and β-carotene yields (4.2 mg. g− 1) were obtained for strain RPFW01.
Rights and permissions
About this article
Cite this article
Parkes, R., Barone, M.E., Herbert, H. et al. Antioxidant Activity and Carotenoid Content Responses of Three Haematococcus sp. (Chlorophyta) Strains Exposed to Multiple Stressors. Appl Biochem Biotechnol 194, 4492–4510 (2022). https://doi.org/10.1007/s12010-022-03926-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-03926-4