Abstract
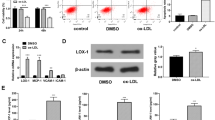
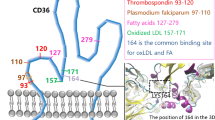
C-reactive protein (CRP) is a well-established biochemical marker for atherosclerosis. Modification of LDL inside the artery wall favors the elevation of this acute phase protein. Hence, this mechanism is considered an important factor to trigger the monocyte to macrophages differentiation which results in the formation of foam cells. Therefore, this key event should be targeted and focused on how this complex (OxLDL + CRP) proceeds to endothelial dysfunction. Oligomeric proanthocyanidins (OPC) is a well-known cardioprotective flavon-3-ols. The present study is challenged between the cardioprotective roles of OPC against the deleterious effect of OxLDL + CRP complex upon endothelial cells. Protein–protein docking was carried out between CRP and LOX-1. This docked protein complex was again docked with OPC to show the inhibitory mechanism of CRP binding with LOX-1. OPC showed a promising inhibitory mechanism against OxLDL + CRP complex. Docking studies showed that in the absence of ligands (OPC), binding of CRP and LOX-1 was greater and vice versa in the presence of ligands. Based on these molecular docking results, in vitro studies have been carried out. The monolayer of endothelial cells was incubated with THP-1 monocytes for 48 h, induced with OxLDL (10 μg/ml) + CRP (15 μg/ml) and cotreated with OPC (100 μg/ml). Morphological changes, cell migration assay, and capillary tube forming assay were carried out. Myeloperoxidase levels were estimated to determine the adhesion of monocytes onto EC monolayer. RT-PCR analysis of L-Selectin was also done. The quantification of NO levels and analysis of mRNA expressions of eNOS was to determine the nitric oxide demand caused due to OxLDL + CRP complex. LOX-1, scavenger receptor levels were analyzed by mRNA expression. Proinflammatory markers such as IL-6, MCP-1, and IL-1β were studied. Accumulation of ROS levels was measured fluorimetrically using DCF-DA staining. Mitochondrial membrane potential was determined by JC-1 dye and cell cycle analysis was done by FACS analysis. To emphasis the results, the OPC-treated group showed decreased levels of proinflammatory markers, LOX-1 and L-selectin levels. Endothelial nitric oxide levels were increased upon OPC treatment and reduction in the ROS levels was also observed. Endothelial cells apoptosis was prevented by OPC. To conclude, OxLDL + CRP complex inhibitory effects of OPC could maintain the normal homeostasis.










Similar content being viewed by others
Data Availability
Yes. Data and results have been generated as part of my doctoral thesis work.
References
Manduteanu, I., & Simionescu, M. (2012). Inflammation in atherosclerosis: A cause or a result of vascular disorders? Journal of cellular and molecular medicine, 16(9), 1978–1990.
Raggi, P., et al. (2018). Role of inflammation in the pathogenesis of atherosclerosis and therapeutic interventions. Atherosclerosis, 276, 98–108.
Badimon, L., Peña, E., Arderiu, G., Padró, T., Slevin, M., Vilahur, G., & Chiva-Blanch, G. (2018). C-reactive protein in atherothrombosis and angiogenesis. Frontiers in Immunology, 9, 430.
Sproston, N. R., & Ashworth, J. J. (2018). Role of C-reactive protein at sites of inflammation and infection. Frontiers in immunology, 9, 754.
Granger, D. N., Senchenkova, E. (2010). Inflammation and the microcirculation. San Rafael (CA): Morgan & Claypool Life Sciences; Chapter 7, Leukocyte–Endothelial Cell Adhesion.
Kany, S., Vollrath, J. T., & Relja, B. (2019). Cytokines in inflammatory disease. International journal of molecular sciences, 20(23), 6008.
Harmon, M. E., Campen, M. J., Miller, C., Shuey, C., Cajero, M., Lucas, S., ... & Lewis, J. (2016). Associations of circulating oxidized LDL and conventional biomarkers of cardiovascular disease in a cross-sectional study of the Navajo population. PloS one, 11(3), e0143102.
Wang, J., Feng, M. J., Zhang, R., Yu, D. M., Zhou, S. J., Chen, R., & Yu, P. (2016). C-reactive protein/oxidized low density lipoprotein/β2-glycoprotein i complexes induce lipid accumulation and inflammatory reaction in macrophages via p38/mitogen-activated protein kinase and nuclear factor-κB signaling pathways. Molecular Medicine Reports, 14(4), 3490–3498.
Yasojima, K., Schwab, C., McGeer, E. G., & McGeer, P. L. (2001). Generation of C-reactive protein and complement components in atherosclerotic plaques. The American Journal of Pathology, 158(3), 1039–1051.
Singh, U., Dasu, M. R., Yancey, P. G., Afify, A., Devaraj, S., & Jialal, I. (2008). Human C-reactive protein promotes oxidized low density lipoprotein uptake and matrix metalloproteinase-9 release in Wistar rats. Journal of Lipid Research, 49(5), 1015–1023.
Rajendran, S., et al. (1996). Effect of tincture of Crataegus on the LDL-receptor activity of hepatic plasma membrane of rats fed an atherogenic diet. Atherosclerosis, 123(1–2), 235–241.
Thirupurasundari, C. J., Jayalakshmi, R., & Devaraj, S. N. (2005). Liver architecture maintenance by tincture of Crataegus against isoproterenol-induced myocardially infarcted rats. Journal of Medicinal Food, 8(3), 400–404.
Elango, C., & Devaraj, S. N. (2010). Immunomodulatory effect of Hawthorn extract in an experimental stroke model. Journal of Neuroinflammation, 7(1), 1–13.
Thiruchenduran, M., Vijayan, N. A., Sawaminathan, J. K., & Devaraj, S. N. (2011). Protective effect of grape seed proanthocyanidins against cholesterol cholic acid diet-induced hypercholesterolemia in rats. Cardiovascular Pathology, 20(6), 361–368.
Mohana, T., Navin, A. V., Jamuna, S., Sadullah, M. S. S., & Devaraj, S. N. (2015). Inhibition of differentiation of monocyte to macrophages in atherosclerosis by oligomeric proanthocyanidins – In-vivo and in-vitro study. Food and Chemical Toxicology, 82(2015), 96–105.
Rathinavel, A., Sankar, J., Sadullah, S. S. M., & Devaraj, S. N. (2018). Oligomeric proanthocyanidins protect myocardium by mitigating left ventricular remodeling in isoproterenol induced post-myocardial infarction. Fundamental and Clinical Pharmacology, 32(1), 51–59.
Sankar, J., Rathinavel, A., Sakeena Sadullah, M. S., Devaraj, S. N. (2018). Oligomeric proanthocyanidins mitigate cholesterol and cholic acid diet–induced hepatic dysfunction in male Sprague Dawley rats. Journal of Biochemical and Molecular Toxicology, e22234.
Jamuna, S., Ashokkumar, R., Sadullah, S. S. M., & Devaraj, S. N. (2019). Oligomeric proanthocyanidins and Epigallocatechin gallate aggravate autophagy of foam cells through the activation of Class III PI3K/Beclin1- complex mediated cholesterol efflux. Biofactors, 45(5), 763–777.
Binkowski, T. A., Naghibzadeh, S., & Liang, J. (2003). CASTp: Computed atlas of surface topography of proteins. Nucleic acids research, 31(13), 3352–3355.
Geldenhuys, W. J., Gaasch, K. E., Watson, M., Allen, D. D., & Vander Schyf, C. J. (2006). Optimizing the use of opensource software applications in drug discovery. Drug Discovery Today, 11(3–4), 127–132.
Duhovny, D., Nussinov, R., Wolfson, H. J. (2002). Efficient unbound docking of rigid molecules. In Gusfield et al. (Eds.), Proceedings of the 2'nd Workshop on Algorithms in Bioinformatics (WABI) Rome, Italy, Lecture Notes in Computer Science (pp. 185–200). Springer Verlag.
Schneidman-Duhovny, D., Inbar, Y., Nussinov, R., & Wolfson, H. J. (2005). PatchDock and SymmDock: Servers for rigid and symmetric docking. Nucleic Acids Research, 33(suppl_2), 363–367.
Jamuna, S., Sadullah, S., Ashokkumar, R., Shanmuganathan, G., & Mozhi, S. S. (2017). Potential antioxidant and cytoprotective effects of essential oil extracted from Cymbopogon citratus on OxLDL and H2O2 LDL induced Human Peripheral Blood Mononuclear Cells (PBMC). Food Science and Human Wellness, 6(2), 60–69.
Bobadilla, A. V. P., Arévalo, J., Sarró, E., Byrne, H. M., Maini, P. K., Carraro, T., ... & Alarcón, T. (2019). In vitro cell migration quantification method for scratch assays. Journal of the Royal Society Interface, 16(151), 20180709.
Kumar, P., Pai, K., Pandey, H. P., & Sundar, S. (2002). NADH-oxidase, NADPH-oxidase and myeloperoxidase activity of visceral leishmaniasis patients. Journal of Medical Microbiology, 51(10), 832–836.
Schulz, K., Kerber, S., & Kelm, M. (1999). Reevaluation of the Griess method for determining NO/NO− 2 in aqueous and protein-containing samples. Nitric Oxide, 3(3), 225–234.
Zhang, C., Yang, F., Zhang, X. W., Wang, S. C., Li, M. H., Lin, L. P., & Ding, J. (2006). Grateloupia longifolia polysaccharide inhibits angiogenesis by downregulating tissue factor expression in HMEC-1 endothelial cells. British Journal of Pharmacology, 148, 741–751.
Talapatra, S. N., Talukdar, P., & Swarnakar, S. (2017). Interaction between C-reactive protein and phytochemical (s) from calotropis procera: An approach on molecular docking. International Letters of Natural Sciences, 61, 43–55.
Shakour, N., Ruscica, M., Hadizadeh, F., Cirtori, C., Banach, M., Jamialahmadi, T., & Sahebkar, A. (2020). Statins and C-reactive protein: In silico evidence on direct interaction. Archives of Medical Science: AMS, 16(6), 1432.
Shakour, N., et al. (2021). Curcumin can bind and interact with CRP: An in silico study. Pharmacological Properties of Plant-Derived Natural Products and Implications for Human Health, 1308, 91–100.
Baldus, S., Eiserich, J. P., Mani, A., Castro, L., Figueroa, M., Chumley, P., ... & Freeman, B. A. (2001). Endothelial transcytosis of myeloperoxidase confers specificity to vascular ECM proteins as targets of tyrosine nitration. The Journal of Clinical Investigation, 108(12), 1759–1770
Csató, V., Pető, A., Fülöp, G. Á., Rutkai, I., Pásztor, E. T., Fagyas, M., ... & Papp, Z. (2015). Myeloperoxidase evokes substantial vasomotor responses in isolated skeletal muscle arterioles of the rat. Acta Physiologica, 214(1), 109–123
Panés, J., Perry, M., & Granger, D. N. (1999). Leukocyte-endothelial cell adhesion: Avenues for therapeutic intervention. British Journal of Pharmacology, 126(3), 537–550.
Gu, B., Dao, L. P., & Wiley, J. (2001). Impaired transendothelial migration of B-CLL lymphocytes: A defect linked to low L-selectin expression. Leukemia & lymphoma, 42(1–2), 5–12.
Strell, C., & Entschladen, F. (2008). Extravasation of leukocytes in comparison to tumor cells. Cell Communication and Signaling : CCS., 6, 10.
Hajjar, D. P., & Gotto, A. M., Jr. (2013). Biological relevance of inflammation and oxidative stress in the pathogenesis of arterial diseases. The American Journal of Pathology, 182(5), 1474–1481.
Forte, M., Conti, V., Damato, A., Ambrosio, M., Puca, A. A., Sciarretta, S., ... & Carrizzo, A. (2016). Targeting nitric oxide with natural derived compounds as a therapeutic strategy in vascular diseases. Oxidative Medicine and Cellular Longevity, 2016
Jin, X., Yi, L., Chen, M. L., Chen, C. Y., Chang, H., Zhang, T., ... & Mi, M. T. (2013). Delphinidin-3-glucoside protects against oxidized low-density lipoprotein-induced mitochondrial dysfunction in vascular endothelial cells via the sodium-dependent glucose transporter SGLT1. PLoS One, 8(7), e68617
Hamoud, S., Hayek, T., Volkova, N., Attias, J., Moscoviz, D., Rosenblat, M., & Aviram, M. (2014). Pomegranate extract (POMx) decreases the atherogenicity of serum and of human monocyte-derived macrophages (HMDM) in simvastatin-treated hypercholesterolemic patients: A double-blinded, placebo-controlled, randomized, prospective pilot study. Atherosclerosis, 232(1), 204–210.
Dandapat, A., Hu, C., Sun, L., & Mehta, J. L. (2007). Small concentrations of oxLDL induce capillary tube formation from endothelial cells via LOX-1–dependent redox-sensitive pathway. Arteriosclerosis, Thrombosis, and Vascular Biology, 27(11), 2435–2442.
Scazzocchio, B., Varì, R., Filesi, C., D’Archivio, M., Santangelo, C., Giovannini, C., ... & Masella, R. (2011). Cyanidin-3-O-β-glucoside and protocatechuic acid exert insulin-like effects by upregulating PPARγ activity in human omental adipocytes. Diabetes, 60(9), 2234–2244
Baba, A. B., Kowshik, J., Krishnaraj, J., Sophia, J., Dixit, M., & Nagini, S. (2016). Blueberry inhibits invasion and angiogenesis in 7, 12-dimethylbenz [a] anthracene (DMBA)-induced oral squamous cell carcinogenesis in hamsters via suppression of TGF-β and NF-κB signaling pathways. The Journal of Nutritional Biochemistry, 35, 37–47.
Xu, L., Choi, T. H., Kim, S., Kim, S. H., Chang, H. W., Choe, M., ... & Zhang, D. (2013). Anthocyanins from black soybean seed coat enhance wound healing. Annals of Plastic Surgery, 71(4), 415–420
Chang, Y. C., Huang, K. X., Huang, A. C., Ho, Y. C., & Wang, C. J. (2006). Hibiscus anthocyanins-rich extract inhibited LDL oxidation and oxLDL-mediated macrophages apoptosis. Food and Chemical Toxicology, 44(7), 1015–1023.
Schnoor, M., Alcaide, P., Voisin, M. B., & van Buul, J. D. (2015). Crossing the vascular wall: Common and unique mechanisms exploited by different leukocyte subsets during extravasation. Mediators of Inflammation, 2015
Yao, Y., & Tsirka, S. E. (2014). Monocyte chemoattractant protein-1 and the blood–brain barrier. Cellular and Molecular Life Sciences, 71(4), 683–697.
Seo, J. W., Yang, E. J., Yoo, K. H., & Choi, I. H. (2015). Macrophage differentiation from monocytes is influenced by the lipid oxidation degree of low density lipoprotein. Mediators of Inflammation, 2015
Mattaliano, M. D., Huard, C., Cao, W., Hill, A. A., Zhong, W., Martinez, R. V., ... & Shih, H. H. (2009). LOX-1-dependent transcriptional regulation in response to oxidized LDL treatment of human aortic endothelial cells. American Journal of Physiology-Cell Physiology, 296(6), C1329–C1337
Hashizume, M., & Mihara, M. (2012). Blockade of IL-6 and TNF-α inhibited oxLDL-induced production of MCP-1 via scavenger receptor induction. European Journal of Pharmacology, 689(1–3), 249–254.
Bode, M., & Mackman, N. (2014). Regulation of tissue factor gene expression in monocytes and endothelial cells: Thromboxane A2 as a new player. Vascular Pharmacology, 62(2), 57–62.
Ou, H. C., Chou, F. P., Sheen, H. M., Lin, T. M., Yang, C. H., & Sheu, W. H. H. (2006). Resveratrol, a polyphenolic compound in red wine, protects against oxidized LDL-induced cytotoxicity in endothelial cells. Clinica Chimica Acta, 364(1–2), 196–204.
Hong, Y. J., Huang, X. M., Liu, X. B., Zhang, C. Y., Zhang, L., & Xu, X. L. (2013). 6-Shogaol protects against oxidized LDL-induced endothelial injruries by inhibiting oxidized LDL-evoked LOX-1 signaling. Evidence-Based Complementary and Alternative Medicine, 2013
Park, K. H., & Park, W. J. (2015). Endothelial dysfunction: Clinical implications in cardiovascular disease and therapeutic approaches. Journal of Korean Medical Science, 30(9), 1213–1225.
Tsai, K. L., Chang, Y. L., Huang, P. H., Cheng, Y. H., Liu, D. H., Chen, H. Y., & Kao, C. L. (2016). Ginkgo biloba extract inhibits oxidized low-density lipoprotein (oxLDL)-induced matrix metalloproteinase activation by the modulation of the lectin-like oxLDL receptor 1-regulated signaling pathway in human umbilical vein endothelial cells. Journal of Vascular Surgery, 63(1), 204–215.
Lubrano, V., & Balzan, S. (2014). LOX-1 and ROS, inseparable factors in the process of endothelial damage. Free Radical Research, 48(8), 841–848.
Ghazi-Khanloosani, M., Bandegi, A. R., Kokhaei, P., Barati, M., & Pakdel, A. (2019). CRP and LOX-1: A mechanism for increasing the tumorigenic potential of colorectal cancer carcinoma cell line. Pathology & Oncology Research, 25(4), 1467–1475.
Zuniga, F. A., Ormazabal, V., Gutierrez, N., Aguilera, V., Radojkovic, C., Veas, C., ... & Aguayo, C. (2014). Role of lectin-like oxidized low density lipoprotein-1 in fetoplacental vascular dysfunction in preeclampsia. BioMed Research International, 2014
Wetterau, E., Stecher, S.-S., Wolff, B., Khouri, S., Staudt, A., Felix, S. B., & Landsberger, M. (2013). Human Endotheliallecitin-Like Oxldlreceptor-1 (Lox-1) Expression Is Notassociated With Impairmentof Endothelium-Dependent Vasoreactivityin Conduitvessels. Journalof Physiologyand Pharmacology, 64(4), 465–472.
Amin, H. P., Czank, C., Raheem, S., Zhang, Q., Botting, N. P., Cassidy, A., & Kay, C. D. (2015). Anthocyanins and their physiologically relevant metabolites alter the expression of IL-6 and VCAM-1 in CD40L and oxidized LDL challenged vascular endothelial cells. Molecular Nutrition & Food Research, 59(6), 1095–1106.
Scheller, J., Chalaris, A., Schmidt-Arras, D., & Rose-John, S. (2011). The pro-and anti-inflammatory properties of the cytokine interleukin-6. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, 1813(5), 878–888.
Wallace, T. C. (2011). Anthocyanins in cardiovascular disease. Advances in Nutrition, 2(1), 1–7.
Amin, H., Czank, C., Cassidy, A., & Kay, C. D. (2012). Effect of cyanidin-glucoside and its metabolites on inflammatory biomarkers of vascular function. Proceedings of the Nutrition Society, 71(OCE2), E55.
Funding
Authors would like to acknowledge the University Grants Commission (UGC), New Delhi, India, for financial support under UGC-BSR meritorious fellowship.
Author information
Authors and Affiliations
Contributions
SJ, AKR, and SND: study conception, experimental design, analysis and interpretation of data. SJ and AKR: drafting the article and SND revising and final approval of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Publish
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Accumulation of OxLDL triggers inflammatory phase during atherosclerosis by elevating the acute phase protein—C-reactive protein.
• Increased levels of CRP and OxLDL could initiate the endothelial dysfunctions via CRP-LOX 1 complex.
• Oligomeric proanthocyanidins isolated from Crataegus oxyacantha berries inhibit the binding of CRP-LOX-1 complex, and possess anti-angiogenic and anti-inflammatory effects.
Rights and permissions
About this article
Cite this article
Jamuna, S., Ashokkumar, R. & Devaraj, S.N. Amelioration of C-Reactive Protein and Lectin Like Oxidized Low Density Lipoprotein Receptor Complex Induced Endothelial Dysfunction by Oligomeric Proanthocyanidins. Appl Biochem Biotechnol 195, 2664–2686 (2023). https://doi.org/10.1007/s12010-021-03792-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-021-03792-6