Abstract
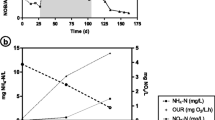
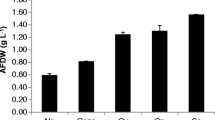
In the present study, the effect of employing the increasing- aeration strategy (IAS) in the oxygen-limited situation and proportionate to increasing oxygen demand of the fungus Schizophyllum commune (S. commune) has been investigated in both stirred tank (STB) and bubble column (BCB) bioreactors. The purpose was to enhance schizophyllan (SPG) production by preventing oxygen starvation, improve mixing conditions of pseudoplastic culture, and intensify shear stress on fungus pellets to release SPG. At first, a constant-aeration rate of 0.08 vvm was implemented in both bioreactors to evaluate the new strategy compared to the previously studied methods. In the second set of experiments with IAS, along with the increasing oxygen demand of culture, the inlet airflow was increased gradually, while the dissolved oxygen (DO) was maintained higher than zero and below 1%. Using IAS in STB significantly raised productivity by about 100% in 96 h from 0.035 to 0.073 g/L.h. Also, employing this strategy in BCB led to a 30% increase in the maximum SPG production from 3.2 to 4.2 g/L. IAS can effectively help handle the operation of S. commune cultivation on a large scale by improving mixing conditions, mass transfer, and shear stress in both bioreactor types. This method had a significant impact on STB cultivation and its productivity so that it can be a practical approach to SPG’s industrial production.








Similar content being viewed by others
Data availability
The data are available from the corresponding author on request.
Abbreviations
- a:
-
Specific interfacial area (m−1)
- BCB:
-
Bubble column bioreactor
- CAS:
-
Constant-aeration strategy
- CW:
-
Cell dry weight, g/L
- \({d}_{b}\) :
-
Bubble diameter (m)
- DL :
-
Diffusion coefficient (m2/s)
- DO:
-
Dissolved oxygen (% air saturation)
- EPS:
-
Extracellular polysaccharides
- g:
-
Gravitational constant (m/s2)
- IAS:
-
Increasing-aeration strategy
- K:
-
Consistency index of broth
- kL :
-
Mass transfer coefficient (s−1)
- P:
-
Power input under gassed condition (W)
- PD:
-
Pellets diameter, mm
- PDA:
-
Potato dextrose agar
- SPG:
-
Schizophyllan
- STB:
-
Stirred tank bioreactor
- t:
-
Time (h)
- Vs :
-
Superficial gas velocity (m/s)
- vvm:
-
Aeration rate, Lair/Lliquid.min
- Yp /s :
-
Product yield coefficient, g/g
- Yx /s :
-
Cell mass yield coefficient, g/g
- \({\alpha }_{r}\) :
-
Apparent yield stress to shear rate ratio
- \(\gamma\) :
-
Shear rate (s−1)
- \({\varepsilon }_{p}\) :
-
Pneumatic energy dissipation rate (W/kg)
- \(\eta\) :
-
Apparent viscosity in power-law (mPa.s)
- \(\phi\) :
-
Gas holdup
- \({\mu }_{c}\) :
-
Viscosity according to the Casson model (Pa. s)
- \(\rho\) :
-
Density (kg/m3)
- \(\tau\) :
-
Shear stress (N/m2)
- \({\tau}_{0}\) :
-
Apparent yield stress (N/m2)
References
Silambarasan, S., Logeswari, P., Cornejo, P., & Kannan, V. R. (2019). Evaluation of the production of exopolysaccharide by plant growth promoting yeast Rhodotorula sp. strain CAH2 under abiotic stress conditions. International Journal of Biological Macromolecules, 121, 55–62.
Sutivisedsak, N., Leathers, T. D., Bischoff, K. M., Nunnally, M. S., & Peterson, S. W. (2013). Novel sources of β-glucanase for the enzymatic degradation of schizophyllan. Enyzme and Microbial Technology, 52, 203–210.
Zhang, Y., Kong, H., Fang, Y., Nishinari, K., & Phillips, G. O. (2013). Schizophyllan: A review on its structure, properties, bioactivities and recent developments. Bioactive Carbohydrates and Dietary Fibre, 1, 53–71.
Safaee-Ardakani, M. R., Hatamian-Zarmi, A., Sadat, S. M., Mokhtari-Hosseini, Z. B., Ebrahimi-Hosseinzadeh, B., Rashidiani, J., & Kooshki, H. (2019). Electrospun Schizophyllan/polyvinyl alcohol blend nanofibrous scaffold as potential wound healing. International Journal of Biological Macromolecules, 127, 27–38.
Jamshidian, H., Shojaosadati, S. A., Vilaplana, F., Mousavi, S. M., & Soudi, M. R. (2016). Characterization and optimization of schizophyllan production from date syrup. International Journal of Biological Macromolecules, 92, 484–493.
Zhong, K., Liu, L., Tong, L., Zhong, X., Wang, Q., & Zhou, S. (2013). Rheological properties and antitumor activity of schizophyllan produced with solid-state fermentation. International Journal of Biological Macromolecules, 62, 13–17.
Yu, Y., Shen, M., Song, Q., & Xie, J. (2018). Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydrate Polymers, 183, 91–101.
Mousaviasl, S., Saleh, T., Shojaosadati, S. A., & Boddohi, S. (2018). Synthesis and characterization of schizophyllan nanogels via inverse emulsion using biobased materials. International Journal of Biological Macromolecules, 120, 468–474.
Rau, U., & Brandt, C. (1994). Oxygen controlled batch cultivations of Schizophyllum commune for enhanced production of branched β-1,3-glucans. Bioprocess Engineering, 11, 161–165.
Castillo, N. A., Valdez, A. L., & Fariña, J. I. (2015). Microbial production of scleroglucan and downstream processing. Frontiers in Microbiology, 6, 1106. https://doi.org/10.3389/fmicb.2015.01106.
Couto, M. R., Gudiña, E. J., Ferreira, D., Teixeira, J. A., & Rodrigues, L. R. (2019). The biopolymer produced by Rhizobium viscosum CECT 908 is a promising agent for application in microbial enhanced oil recovery. New Biotechnology, 49, 144–150.
Kumar, M. S., & Singlial, R. S. (2011). Rheological behavior of schizophyllan in fermentation system. American Journal of Food Technology, 6, 781–789.
Rau, U., Gura, E., Olszewski, E., & Wagner, F. (1992). Enhanced glucan formation of filamentous fungi by effective mixing, oxygen limitation and fed-batch processing. Journal of Industrial Microbiology, 9, 19–25.
Gura, E., & Rau, U. (1993). Comparison of agitators for the production of branched β-1,3-d-glucans by Schizophyllum commune. Journal of Biotechnology, 27, 193–201.
Wawra, S., Fesel, P., Widmer, H., Neumann, U., Lahrmann, U., Becker, S., Hehemann, J. H., Langen, G., & Zuccaro, A. (2019). FGB1 and WSC3 are in planta-induced β-glucan-binding fungal lectins with different functions. New Phytologist, 222, 1493–1506.
Jegatheeswaran, S., Kazemzadeh, A., & Ein-Mozaffari, F. (2019). Enhanced aeration efficiency in non-Newtonian fluids using coaxial mixers: High-solidity ratio central impeller with an anchor. Chemical Engineering Journal, 378, 122081.
Shu, C. H., Chou, P. F., & Hsu, I. C. (2005). Effects of morphology and oxygen supply on schizophyllan formation by Schizophyllum commune using a pellet size controlling bioreactor. Journal of Chemical Technology and Biotechnology, 80, 1383–1388.
Doran, P. M. (2012). Bioprocess engineering principles (2nd ed.). Elsevier Ltd.
Papagianni, M. (2004). Fungal morphology and metabolite production in submerged mycelial processes. Biotechnology Advances, 22, 189–259.
Veiter, L., Rajamanickam, V., & Herwig, C. (2018). The filamentous fungal pellet—Relationship between morphology and productivity. Applied Microbiology and Biotechnology, 102, 2997–3006.
Satari, B., Karimi, K., Taherzadeh, M. J., & Zamani, A. (2016). Co-production of fungal biomass derived constituents and ethanol from citrus wastes free sugars without auxiliary nutrients in airlift bioreactor. International Journal of Molecular Sciences, 17, 302.
Schmid, J., Meyer, V., & Sieber, V. (2011). Scleroglucan: Biosynthesis, production and application of a versatile hydrocolloid. Applied Microbiology and Biotechnology, 91, 937–947.
Wucherpfennig, T., Kiep, K. A., Driouch, H., Wittmann, C., & Krull, R. (2010). Morphology and rheology in filamentous cultivations. Advances in Applied Microbiology, 72, 89–136. https://doi.org/10.1016/S0065-2164(10)72004-9.
Kang, X., Wang, H., Wang, Y., Harvey, L. M., & McNeil, B. (2001). Hydrodynamic characteristics and mixing behaviour of Sclerotium glucanicum culture fluids in an airlift reactor with an internal loop used for scleroglucan production. Journal of Industrial Microbiology and Biotechnology, 27, 208–214.
Ansari, S., Jalili, H., Bizukojc, M., & Amrane, A. (2019). Influence of the construction of porous spargers on lovastatin production by Aspergillus terreus ATCC 20,542 in a laboratory bubble column. Bioprocess and Biosystems Engineering. https://doi.org/10.1007/s00449-019-02118-5
Rau U. (1999) Production of Schizophyllan. In C. Bucke (Ed.), Carbohydrate biotechnology protocols. Methods in biotechnology (vol. 10, pp. 43–55). Humana Press. https://doi.org/10.1007/978-1-59259-261-6_4.
Steiner, W., Lafferty, R. M., Gomes, I., & Esterbauer, H. (1987). Studies on a wild strain of Schizophyllum commune: Cellulase and xylanase production and formation of the extracellular polysaccharide Schizophyllan. Biotechnology and Bioengineering, 30, 169–178.
Mohammadi, A., Shojaosadati, S. A., Tehrani, H. J., Mousavi, S. M., Saleh, T., & Khorasani, A. C. (2018). Schizophyllan production by newly isolated fungus Schizophyllum commune IBRC-M 30213: Optimization of culture medium using response surface methodology. Annales de Microbiologie, 68, 47–62.
Saptoro, A., Herng, M. T. H., & Teng, E. L. W. (2014). Oxygen transfer to cassava starch solutions in an aerated, well-mixed bioreactor: Experimental and mass transfer studies. Korean Journal of Chemical Engineering, 31, 650–658.
Garcia-Ochoa, F., & Gomez, E. (2004). Theoretical prediction of gas-liquid mass transfer coefficient, specific area and hold-up in sparged stirred tanks. Chemical Engineering Science, 59, 2489–2501.
Papapostolou, A., Karasavvas, E., & Chatzidoukas, C. (2019). Oxygen mass transfer limitations set the performance boundaries of microbial PHA production processes – A model-based problem investigation supporting scale-up studies. Biochemical Engineering Journal, 148, 224–238.
Kelly, S., Grimm, L. H., Bendig, C., Hempel, D. C., & Krull, R. (2006). Effects of fluid dynamic induced shear stress on fungal growth and morphology. Process Biochemistry, 41, 2113–2117.
Garcia-Ochoa, F., & Gomez, E. (2009). Bioreactor scale-up and oxygen transfer rate in microbial processes: An overview. Biotechnology Advances, 27, 153–176.
Teoh, Y. P., & Don, M. M. (2012). Optimization of parameters for mycelia growth by Schizophyllum commune and a kinetic model study of its growth morphology. Journal of Applied Sciences, 12, 1100–1105.
Van Bodegom, P. (2007). Microbial maintenance: A critical review on its quantification. Microbial Ecology, 53, 513–523.
Mahnke, E. U., Büsclier, K., & Hempel, D. C. (2000). A novel approach for the determination of mechanical stresses in gas-liquid reactors. Chemical Engineering and Technology, 23, 509–513.
Funding
This work was financially supported by Tarbiat Modares University, Tehran, Iran.
Author information
Authors and Affiliations
Contributions
Kiyana Saeedian: Investigation, formal analysis, writing—original draft preparation, review and editing.
Seyed Abbas Shojaosadati: Supervision, funding acquisition, writing—review and editing.
Seyed Morteza Zamir: Advice, writing—review and editing.
Aref Mohammadi: Investigation.
All authors approved the publication of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Stirred tank bioreactor (STB) cultivation with the constant-aeration rate of 0.08 vvm; 6th hour: no significant difference was visible (MP4 7224 KB)
Stirred tank bioreactor (STB) cultivation with the constant-aeration rate of 0.08 vvm; 144th hour: By sedimentation of the pellets, the air dissolution decreased so that air channels occurred in bioreactor and the air bubbles transferred through channels made foam (MP4 4818 KB)
Stirred tank bioreactor (STB) cultivation with the increasing-aeration strategy (IAS); 96th hour: better mixing condition than constant-aeration strategy (CAS), starting pellets destruction (MP4 9658 KB)
Stirred tank bioreactor (STB) cultivation with the increasing-aeration strategy (IAS); 120th hour: cell fragments formation and cell contents release, undissolved air and foam formation (MP4 4271 KB)
Bubble column bioreactor (BCB) cultivation with the constant-aeration rate of 0.08 vvm; 4th hour: transparent medium with no significant difference (MP4 5452 KB)
Bubble column bioreactor (BCB) cultivation with the constant-aeration rate of 0.08 vvm; 168th hour: concentrated medium with settled pellets and air channels (MP4 4678 KB)
Bubble column bioreactor (BCB) cultivation with the increasing-aeration strategy (IAS); 168th hour: better mixing condition than constant-aeration strategy (CAS), pellets destruction, and cell fragment formation (MP4 4678 KB)
Rights and permissions
About this article
Cite this article
Saeedian, K., Shojaosadati, S.A., Zamir, S.M. et al. Increasing-Aeration Strategy: a Practical Approach to Enhance the Schizophyllan Production and Improve the Operational Conditions of Schizophyllum commune Cultivation in the Stirred Tank and Bubble Column Bioreactors. Appl Biochem Biotechnol 194, 2284–2300 (2022). https://doi.org/10.1007/s12010-021-03777-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-021-03777-5