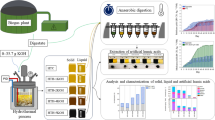
Abstract
Macroalgae are an important source of food, fertilizer, hydrocolloids, and healthful bioactive components. Macroalgae are also being considered sources of biofuels, which require minimal demands for arable land, fresh water, or fertilizers. In this study, we explored the possibility of developing a red seaweed biorefinery process to extract carrageenan while producing chemical or biofuel co-products derived from the carrageenan extraction wastes. A common approach to processing organic wastes is to generate biogas; however, in this study, we targeted a potentially higher value option by applying acidogenic digestion to convert extraction wastes to carboxylic acids and derived compounds. Using an open culture of microorganisms, wastes from a carrageenan extraction plant were converted to mixed carboxylic acids, which were then neutralized and thermally decomposed to a variety of ketones. Batch digestions of the wastes were carried out at temperatures of 35 °C and 55 °C. Either calcium carbonate or ammonium bicarbonate was used as buffer. A solid–liquid counter-current percolation fermentation was operated in four stages at 35 °C. Digestion produced carboxylic acids ranging in chain length from one to seven carbons. The mesophilic temperature gave higher carboxylic acid yield and longer chain acids, with the highest acid titer reaching 18 g L−1. Thermal decomposition of carboxylate salts produced a mixture of ketones which contained acetone, 3-pentanone, 2-hexanone, 2-heptanone, 3-heptanone, and 4-octanone as major products. These ketones could be sold as chemicals or hydrogenated to form corresponding chain length secondary alcohols which deliver higher energy density than ethanol.
Graphical abstract











Similar content being viewed by others
Data Availability
No additional data to present.
Code Availability
Not applicable.
References
Wei, N., Quarterman, J., & Jin, Y. S. (2013). Marine macroalgae: An untapped resource for producing fuels and chemicals. Trends in biotechnology, 31(2), 70–77.
Ghadiryanfar, M., Rosentrater, K. A., Keyhani, A., & Omid, M. (2016). A review of macroalgae production, with potential applications in biofuels and bioenergy. Renewable and Sustainable Energy Reviews, 54, 473–481.
Gao, K., & McKinley, K. R. (1994). Use of macroalgae for marine biomass production and CO2 remediation: A review. Journal of Applied Phycology, 6(1), 45–60.
John, R. P., Anisha, G. S., Nampoothiri, K. M., & Pandey, A. (2011). Micro and macroalgal biomass: A renewable source for bioethanol. Bioresource technology, 102(1), 186–193.
Stiger-Pouvreau, V., Bourgougnon, N., & Deslandes, E. (2016). Carbohydrates from seaweeds. Seaweed in health and disease prevention (pp. 223–274). Academic Press.
Goh, C. S., & Lee, K. T. (2010). A visionary and conceptual macroalgae-based third-generation bioethanol (TGB) biorefinery in Sabah, Malaysia as an underlay for renewable and sustainable development. Renewable and Sustainable Energy Reviews, 14(2), 842–848.
Vergara-Fernández, A., Vargas, G., Alarcón, N., & Velasco, A. (2008). Evaluation of marine algae as a source of biogas in a two-stage anaerobic reactor system. Biomass and Bioenergy, 32, 338–344.
Anjaneyulu, K., Tarwadi, S. J., & Mehta, D. J. (1989). Anaerobic digestion of seaweed for biogas: A kinetic evaluation. Journal of Chemical Technology and Biotechnology, 45, 5–14.
Milledge, J. J., Nielsen, B. V., Maneein, S., & Harvey, P. J. (2019). A brief review of anaerobic digestion of algae for bioenergy. Energies, 12(6), 1166.
Maia, M. R. G., Fonseca, A. J. M., Oliveira, H. M., Mendonça, C., & Cabrita, A. R. J. (2016). The potential role of seaweeds in the natural manipulation of rumen fermentation and methane production. Scientific Reports, 6, 32321.
Abbott, D. W., Aasen, I. M., Beauchemin, K. A., Grondahl, F., Gruninger, R., Hayes, M., Huws, S., Kenny, D. A., Krizsan, S. J., Kirwan, S. F., Lind, V., Meyer, U., Ramin, M., Theodoridou, K., von Soosten, D., Walsh, P. J., Waters, S., & Xing, X. (2020). Seaweed and seaweed bioactives for mitigation of enteric methane: Challenges and opportunities. Animals, 10, 2432.
Agler, M. T., Wrenn, B. A., Zinder, S. H., & Angenent, L. T. (2011). Waste to bioproduct conversion with undefined mixed cultures: The carboxylate platform. Trends in Biotechnology, 29(2), 70–78.
Baddam, R., & van Walsum, G. P. (2017). Acidogenic digestion of pre-pulping extracts for production of fuels and bioproducts via carboxylate platform processing. Applied Biochemistry and Biotechnology, 182(3), 1076–1094.
Bastidas-Oyanedel, Juan-Rodrigo., Bonk, Fabian, Thomsen, Mette Hedegaard, & Schmidt, Jens Ejbye. (2015). Dark fermentation biorefinery in the present and future (bio)chemical industry. Reviews in Environmental Science and Biotechnology. https://doi.org/10.1007/s11157-015-9369-3
Álvarez-Viñas, M., Flórez-Fernández, N., Torres, M. D., & Domínguez, H. (2019). Successful approaches for a red seaweed biorefinery. Marine Drugs, 17(11), 620.
Ingle, K., Vitkin, E., Robin, A., Yakhini, Z., Mishori, D., & Golberg, A. (2018). Macroalgae biorefinery from Kappaphycusalvarezii: Conversion modeling and performance prediction for India and Philippines as examples. BioEnergy Research, 11, 22–32.
Holtzapple, M. T., Davison, R. R., Ross, M. K., Aldrett-Lee, S., Nagwani, M., Lee, C. M., Lee, C., Adelson, S., Kaar, W., Gaskin, D., Shirage, H., Chang, N. S., Chang, V. S., & Loescher, M. E. (1999). Biomass conversion to mixed alcohol fuels using the MixAlco process. Applied Biochemistry and Biotechnology, 77–79, 609–631.
Holtzapple, M. T., & Granda, C. B. (2009). Carboxylate platform: The MixAlco process Part 1: Comparison to three biomass conversion platforms. Applied Biochemistry and Biotechnology, 156(1–3), 95–106.
Steinbüchel, A., & Rhee, S. K. (2005). Polysaccharides and polyamides in the food industry. Wiley-VCH Verlag, GmbH & Co.
McHugh, D. J. (2003) A guide to the seaweed industry, FAO Fisheries Technical Paper 441. Food and Agriculture Organization of the United Nations, Rome. < http://www.fao.org/DOCREP/006/Y4765E/y4765e00.htm> accessed on June 2021.
Thanakoses, P., Black, A. S., & Holtzapple, M. T. (2003). Fermentation of corn stover to carboxylic acids. Biotechnology and Bioengineering, 83(2), 191–200.
Blackman, E. D., & van Walsum, G. P. (2009). Production of renewable bioproducts and reduction of phosphate pollution through the lime pretreatment and acidogenic digestion of dairy manure. Environmental Progress and Sustainable Energy, 28, 121–133.
Pham, V., Holtzapple, M., & EI-Halwagi, M. (2010). Techno-economic analysis of biomass to fuel conversion via the MixAlco process. Journal of Industrial Microbiology and Biotechnology, 37(11), 1157–1168.
Engelberth, A. S., Wheeler, M. C., & van Walsum, G. P. (2018). Techno-Economic comparison of three scenarios for upgrading a hemicellulose-rich pre-pulping extract to mixed-alcohols. Biofuels, Bioproducts and Biorefining, 12(6), 1082–1094.
Ross, M. K., & Holtzapple, M. T. (2001). Laboratory method for high-solids countercurrent fermentations. Applied Biochemistry and Biotechnology, 94(2), 111–126.
Sluiter, A., Hames, B., Hyman, D., Payne, C., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., & Wolfe, J. (2008) Determination of total solids in biomass and total dissolved solids in liquid process samples: Laboratory Analytical Procedure (LAP). Technical Report NREL/TP-510–42621, National Renewable Energy Laboratory, Colorado.
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., & Templeton, D. (2008) Determination of ash in biomass: Laboratory Analytical Procedure (LAP). Technical Report NREL/TP-510–42622, National Renewable Energy Laboratory, Colorado.
Aiello-Mazzarri, C., Agbogbo, F. K., & Holtzapple, M. T. (2006). Conversion of municipal solid waste to carboxylic acids using mixed culture of mesophilic microorganisms. Bioresource Technology, 97, 47–56.
Agbogbo, F. K. (2005) Anaerobic fermentation of rice straw and chicken manure to carboxylic acids. Ph.D. Dissertation, Texas A&M University, Texas.
EI-Ssarraf, W. M., & EI-Shaarawy, G. (1994). Chemical composition of some marine algae from the mediterranean sea of Alexandria, Egypt. The Bulletin of the High Institute of Public Health, 24, 3.
Thanakoses, P., (2002) Conversion of bagasse and corn stover to mixed carboxylic acids using a mixed culture of mesophilic microorganisms. Ph.D. Dissertation, Texas A&M University, Texas.
Cui, S. W. (2005). Food carbohydrates: Chemistry, physical properties, and applications. CRC Press, Taylor & Francis Group.
Bonk, F., Bastidas-Oyanedel, J.-R., Yousef, A. F., & Schmidt, J. E. (2017). Exploring the selective lactic acid production from food waste in uncontrolled pH mixed culture fermentations using different reactor configurations. Bioresource Technology, 238(2017), 416–424.
Bhatia, M. (2011) Thermal conversion of carboxylate salts and catalytic ketone hydrogenation. M.S. Thesis, University of Maine, Orono, Maine.
Granda, C., Holtzapple, M., Luce, G., Searcy, K., & Mamrosh, D. (2009). Carboxylate platform: The MixAlco process part 2: Process economics. Applied Biochemistry and Biotechnology, 156, 107–124.
Acknowledgements
We appreciate the support from FMC Biopolymer Rockland, Maine, who provided the Algefiber®. We thank Dr. Martin Lawoko, Dr. Sedat Beis, Dr. Diane Smith, Dr. Byung-Hwan Um, Keith Hodgins, Nick Hill, Justin Crouse, and Amos Cline for the analytical and technical support. Special thanks to Dr. Adriaan R.P van Heiningen and Dr. Clayton Wheeler for their insight and guidance.
Funding
US Department of Energy grant No. DE-FG36-08GO18165 provided the funding for this research.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by S. A. Karunarathne, overseen by G. P. van Walsum. The first draft of the manuscript was written by S. A. Karunarathne and all authors contributed to previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Karunarathne and van Walsum both consent to the publication of this material.
Competing interests
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• By-products from seaweed processing are suitable for carboxylate platform processing.
• Carrageenan by-product solids were converted to mixed carboxylic acids and ketones.
• Fermentation at mesophilic temperatures with CaCO3 buffer gave optimal acid yields.
• Four percolation columns in series demonstrated countercurrent fermentation.
Rights and permissions
About this article
Cite this article
Karunarathne, S.A., van Walsum, G.P. Integrating the Carboxylate Platform into a Red Seaweed Biorefinery. Appl Biochem Biotechnol 194, 1235–1258 (2022). https://doi.org/10.1007/s12010-021-03699-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-021-03699-2