Abstract
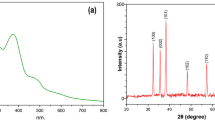
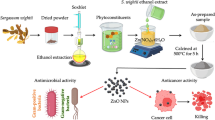
The present study highlights/demonstrates facile synthesis of β-Glucan nanoparticles (β-GluNPs) that can be used in the prevention of breast cancer and other infectious diseases. Moreover, this method is inexpensive and shows effectivity towards different biological applications. Further, the characterization of synthesized β-GluNPs was exclusively confirmed through UV–Vis spectroscopy, Fourier transform infrared spectroscopy (FT-IR), dynamic light scattering (DLS), zeta potential, scanning electron microscopy (SEM), high resolution-transmission electron microscopy (HR-TEM), and X-ray powder diffraction (XRD) analysis. The synthesized β-GluNPs were further confirmed by FT-IR spectroscopy. The HR-TEM results demonstrated that the formation of polydispersed nanoparticles with a mean size of 20 ± 5 nm. The hydrostatic zeta potential was − 22.7 mV, which indicated their colloidal stability. The XRD pattern revealed the crystalline nature of the nanoparticles. Besides, β-GluNPs showed better antibacterial activity against the tested pathogens. The apoptosis and DNA fragmentation observed to be IC50 42.5 µg/ml of the β-GluNPs. The DNA fragmentation assay indicated the selective inhibition of the MCF-7 cell line by DNA damage. Hence, the study reports that the β-GluNPs have a potential to be used as a promising alternative drug against human breast cancer.




Similar content being viewed by others
Data Availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Gajendiran, M., Choi, J., Kim, S. J., Kim, K., Shin, H., Koo, H. J., & Kim, K. (2017). Conductive biomaterials for tissue engineering applications. Journal of Industrial and Engineering Chemistry. https://doi.org/10.1016/j.jiec.2017.02.031
Esparza-Soto, M., & Westerhoff, P. (2003). Biosorption of humic and fulvic acids to live activated sludge biomass. Water Research, 37(10), 2301–2310. https://doi.org/10.1016/S0043-1354(02)00630-9
Liu, J., Qin, G., Raveendran, P., & Ikushima, Y. (2006). Facile “green” synthesis, characterization, and catalytic function of β-D-glucose-stabilized Au nanocrystals. Chemistry - A European Journal, 12(8), 2131–2138. https://doi.org/10.1002/chem.200500925
Balantrapu, K., & Goia, D. V. (2009). Silver nanoparticles for printable electronics and biological applications. Journal of Materials Research, 24(9), 2828–2836. https://doi.org/10.1557/jmr.2009.0336
Li, J., Cai, C., Yang, C., Li, J., Sun, T., & Yu, G. (2019). Recent advances in pharmaceutical potential of brown algal polysaccharides and their derivatives. Current Pharmaceutical Design, 25(11), 1290–1311. https://doi.org/10.2174/1381612825666190618143952
Husemann, E. (1968). Book review: Chemistry and enzymology of marine algal polysaccharides. By E. Percival and R. H. McDowell. Angewandte Chemie International Edition in English, 7(9), 746–746. https://doi.org/10.1002/anie.196807463
Aoe, S., Ichinose, Y., Kohyama, N., Komae, K., Takahashi, A., Abe, D., …, Yanagisawa, T. (2017). Effects of high β-glucan barley on visceral fat obesity in Japanese individuals: A randomized, double-blind study. Nutrition, 42. https://doi.org/10.1016/j.nut.2017.05.002
Vetvicka, V., Vannucci, L., Sima, P., & Richter, J. (2019). Beta glucan: Supplement or drug? From laboratory to clinical trials. Molecules. https://doi.org/10.3390/molecules24071251
Sathiyabama, M., & Parthasarathy, R. (2016). Biological preparation of chitosan nanoparticles and its in vitro antifungal efficacy against some phytopathogenic fungi. Carbohydrate Polymers, 151. https://doi.org/10.1016/j.carbpol.2016.05.033.
Sathiyabama, M., & Manikandan, A. (2016). Chitosan nanoparticle induced defense responses in fingermillet plants against blast disease caused by Pyricularia grisea (Cke.) Sacc. Carbohydrate Polymers, 154, 241–246. https://doi.org/10.1016/j.carbpol.2016.06.089
Anusuya, S., & Sathiyabama, M. (2014). Preparation of β-d-glucan nanoparticles and its antifungal activity. International Journal of Biological Macromolecules, 70. https://doi.org/10.1016/j.ijbiomac.2014.07.011.
Sokolova, R. V., Ermakova, S. P., Awada, S. M., Zvyagintseva, T. N., & Kanaan, H. M. (2011). Composition, structural characteristics, and antitumor properties of polysaccharides from the brown algae Dictyopteris polypodioides and Sargassum sp. Chemistry of Natural Compounds, 47(3), 329–334. https://doi.org/10.1007/s10600-011-9925-1
Daou, C., & Zhang, H. (2012). Oat Beta-Glucan: Its role in health promotion and prevention of diseases. Comprehensive Reviews in Food Science and Food Safety, 11(4), 355–365. https://doi.org/10.1111/j.1541-4337.2012.00189.x
Liang, J., Melican, D., Cafro, L., Palace, G., Fisette, L., Armstrong, R., & Patchen, M. L. (1998). Enhanced clearance of a multiple antibiotic resistant Staphylococcus aureus in rats treated with PGG-glucan is associated with increased leukocyte counts and increased neutrophil oxidative burst activity. International Journal of Immunopharmacology, 20(11), 595–614. https://doi.org/10.1016/S0192-0561(98)00007-1
Kaiser, A. B., & Kernodle, D. S. (1998). Synergism between poly-(1-6)-β-D-glucopyranosyl-(1-3)-β-D- glucopyranose glucan and cefazolin in prophylaxis of staphylococcal wound infection in a guinea pig model. Antimicrobial Agents and Chemotherapy, 42(9), 2449–2451. https://doi.org/10.1128/AAC.42.9.2449
Das, A., Salloum, F. N., Xi, L., Rao, Y. J., & Kukreja, R. C. (2009). ERK phosphorylation mediates sildenafil-induced myocardial protection against ischemia-reperfusion injury in mice. American Journal of Physiology-Heart and Circulatory Physiology, 296(5), H1236–H1243. https://doi.org/10.1152/ajpheart.00100.2009
Misra, R., Acharya, S., & Sahoo, S. K. (2010). Cancer nanotechnology: Application of nanotechnology in cancer therapy. Drug Discovery Today. https://doi.org/10.1016/j.drudis.2010.08.006
Farnsworth, N. R., & Soejarto, D. D. (1985). Potential consequence of plant extinction in the United States on the current and future availability of prescription drugs. Economic Botany, 39(3), 231–240. https://doi.org/10.1007/BF02858792
Nakkala, J. R., Mata, R., Gupta, A. K., & Sadras, S. R. (2014). Biological activities of green silver nanoparticles synthesized with Acorous calamus rhizome extract. European Journal of Medicinal Chemistry, 85. https://doi.org/10.1016/j.ejmech.2014.08.024.
Parveen, S., & Sahoo, S. K. (2008). Polymeric nanoparticles for cancer therapy. Journal of Drug Targeting. https://doi.org/10.1080/10611860701794353
Singhal, S., Nie, S., & Wang, M. D. (2010). Nanotechnology applications in surgical oncology. Annual Review of Medicine. https://doi.org/10.1146/annurev.med.60.052907.094936
Yezhelyev, M. V., Gao, X., Xing, Y., Al-Hajj, A., Nie, S., & O’Regan, R. M. (2006). Emerging use of nanoparticles in diagnosis and treatment of breast cancer. Lancet Oncology. https://doi.org/10.1016/S1470-2045(06)70793-8
Sathishkumar, G., Bharti, R., Jha, P. K., Selvakumar, M., Dey, G., Jha, R., …, Sivaramakrishnan, S. (2015). Dietary flavone chrysin (5,7-dihydroxyflavone ChR) functionalized highly-stable metal nanoformulations for improved anticancer applications. RSC Advances, 5(109). https://doi.org/10.1039/c5ra15060d.
Yugay, Y. A., Usoltseva, R. V., Silant’ev, V. E., Egorova, A. E., Karabtsov, A. A., Kumeiko, V. V., & Shkryl, Y. N. (2020). Synthesis of bioactive silver nanoparticles using alginate, fucoidan and laminaran from brown algae as a reducing and stabilizing agent. Carbohydrate Polymers, 245. https://doi.org/10.1016/j.carbpol.2020.116547.
Exbrayat, J. M., Moudilou, E. N., & Lapied, E. (2015). Harmful effects of nanoparticles on animals. Journal of Nanotechnology. https://doi.org/10.1155/2015/861092
Ghormade, V., Deshpande, M. V., & Paknikar, K. M. (2011). Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnology Advances. https://doi.org/10.1016/j.biotechadv.2011.06.007
Parthasarathy, R., Chandrika, M., Yashavantha Rao, H. C., Kamalraj, S., Jayabaskaran, C., & Pugazhendhi, A. (2020). Molecular profiling of marine endophytic fungi from green algae: Assessment of antibacterial and anticancer activities. Process Biochemistry, 96. https://doi.org/10.1016/j.procbio.2020.05.012.
Kim, Y. T., Kim, E. H., Cheong, C., Williams, D. L., Kim, C. W., & Lim, S. T. (2000). Structural characterization of β-d-(1→3, 1→6)-linked glucans using NMR spectroscopy. Carbohydrate Research, 328(3), 331–341. https://doi.org/10.1016/S0008-6215(00)00105-1
Zlatkovic, D., Jakovljevic, D., Zekovic, D., & Vrvic, M. (2003). A glucan from active dry baker’s yeast (Saccharomyces cerevisiae): A chemical and enzymatic investigation of the structure. Journal of the Serbian Chemical Society, 68(11), 805–809. https://doi.org/10.2298/JSC0311805Z
Gidley, M., & Grant Reid, J. (2006). Galactomannans and other cell wall storage polysaccharides in seeds. Food Polysaccharides and Their Applications. https://doi.org/10.1201/9781420015164.ch6
Mathlouthi, M., & Koenig, J. L. (1987). Vibrational spectra of carbohydrates. Advances in Carbohydrate Chemistry and Biochemistry, 44(C). https://doi.org/10.1016/S0065-2318(08)60077-3.
Wu, Y., Yang, W., Wang, C., Hu, J., & Fu, S. (2005). Chitosan nanoparticles as a novel delivery system for ammonium glycyrrhizinate. International Journal of Pharmaceutics, 295(1–2), 235–245. https://doi.org/10.1016/j.ijpharm.2005.01.042
Khlebtsov, B. N., & Khlebtsov, N. G. (2011). On the measurement of gold nanoparticle sizes by the dynamic light scattering method. Colloid Journal, 73(1), 118–127. https://doi.org/10.1134/S1061933X11010078
Qi, L., Xu, Z., Jiang, X., Xu, C., & Zou, X. (2004). Preparation and antibacterial activity of chitosan nanoparticles. Carbohydrate Research, 339(16), 2693–2700. https://doi.org/10.1016/j.carres.2004.09.007
Tripathi, S., Mehrotra, G. K., & Dutta, P. K. (2009). Physicochemical and bioactivity of cross-linked chitosan–PVA film for food packaging applications. International Journal of Biological Macromolecules, 45(4), 372–376. https://doi.org/10.1016/j.ijbiomac.2009.07.006
Piani, L., & Papo, A. (2013). Sodium tripolyphosphate and polyphosphate as dispersing agents for alumina suspensions: Rheological characterization. Journal of Engineering (United Kingdom), 2013.https://doi.org/10.1155/2013/930832.
Shrivastava, S., Bera, T., Roy, A., Singh, G., Ramachandrarao, P., & Dash, D. (2007). Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology. https://doi.org/10.1088/0957-4484/18/22/225103
Zarina, A., & Nanda, A. (2014). Green approach for synthesis of silver nanoparticles from marine streptomyces- MS 26 and their antibiotic efficacy. Journal of Pharmaceutical Sciences and Research, 6(10), 321.
Nel, A., Xia, T., Mädler, L., & Li, N. (2006). Toxic potential of materials at the nanolevel. Science. 311(5761), 622-627. https://doi.org/10.1126/science.1114397
Chan, W. K., Cheung, C. C. H., Law, H. K. W., Lau, Y. L., & Chan, G. C. F. (2008). Ganoderma lucidum polysaccharides can induce human monocytic leukemia cells into dendritic cells with immuno-stimulatory function. Journal of Hematology and Oncology, 1(1), 1-12. https://doi.org/10.1186/1756-8722-1-9.
Kidd, P. M. (2000). The use of mushroom glucans and proteoglycans in cancer treatment. Alternative Medicine Review, 5(1), 4-27.
Li, Q. F., Shi, S. L., Liu, Q. R., Tang, J., Song, J., & Liang, Y. (2008). Anticancer effects of ginsenoside Rg1, cinnamic acid, and tanshinone IIA in osteosarcoma MG-63 cells: Nuclear matrix downregulation and cytoplasmic trafficking of nucleophosmin. International Journal of Biochemistry and Cell Biology, 40(9), 1918–1929. https://doi.org/10.1016/j.biocel.2008.01.031
Ott, M., Gogvadze, V., Orrenius, S., & Zhivotovsky, B. (2007). Mitochondria, oxidative stress and cell death. Apoptosis. https://doi.org/10.1007/s10495-007-0756-2
Markovic, Z. M., Harhaji-Trajkovic, L. M., Todorovic-Markovic, B. M., Kepić, D. P., Arsikin, K. M., Jovanović, S. P., …, Trajkovic, V. S. (2011). In vitro comparison of the photothermal anticancer activity of graphene nanoparticles and carbon nanotubes. Biomaterials, 32(4). https://doi.org/10.1016/j.biomaterials.2010.10.030.
Funding
Parthasarathy Ramalingam (RP) received a research grant from DST-SERB, Govt of India, New Delhi, for the National Post-Doctoral fellowship (Ref. no. Dr. R. Parthasarathy Ramalingam PDF/2017/001184); Seetharaman Prabu Kumar (S.P.K.) received funding from Laboratory of Functional Molecules and Materials, School of Physics and Optoelectronic Engineering, Shandong University of Technology, Zibo 255000, China; H.C. Yashavantha Rao received UGC Kothari fellowship, New Delhi (No. F.4_2/2006 (BSR)/BL/17–18/0234); The authors also received support from confocal microscopy central facility, Department of Biochemistry, Division of Biological Sciences, Indian Institute of Science (IISc), Bangalore.
Author information
Authors and Affiliations
Contributions
Conceptualization of this research, design of experiments (R.P., S.P.K., and H.C.Y.R); Extraction of β-Glucan and synthesis, characterization of β-GluNPs (R.P., S.P.K., and H.C.Y.R); Performance of antibacterial and anticancer studies (R.P.); Drafting of the manuscript, (R.P. and S.P.K.); Writing–original draft preparation, supervision, proofreading, and manuscript design, (R.P., S.P.K., and J.C.); Funding acquisition (R.P., S.P.K., and J.C.). All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Parthasarathy, R., Kumar, S.P., Rao, H.C.Y. et al. Synthesis of β-Glucan Nanoparticles from Red Algae–Derived β-Glucan for Potential Biomedical Applications. Appl Biochem Biotechnol 193, 3983–3995 (2021). https://doi.org/10.1007/s12010-021-03674-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-021-03674-x