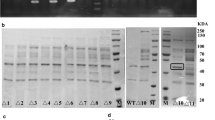
Abstract
Bacillus subtilis has many attributes that make it a popular host for recombinant protein production. Although its protein production ability has been enhanced through protease gene disruption, residual proteases like quality control HtrA and HtrB can limit protein yield by degrading inadequately folded proteins present during overexpression. In this study, two strategies were employed to increase production of industrial enzyme pullulanase: enhancing extracellular pullulanase folding and optimizing its signal peptide. The hypothesis was that disruption of dltB gene of expression host B. subtilis WS9 would enhance recombinant extracellular folding by increasing cation binding to the cell’s outer envelope. Consistent with this hypothesis, disrupting dltB enhanced pullulanase production by 49% in shake-flask cultures. The disruption also enhanced extracellular α-CGTase and β-CGTase production by 25% and 35%, respectively. Then, more effective signal peptide for pullulanase production was identified through high-throughput screening of 173 unique B. subtilis signal peptides. Replacing the native signal peptide of pullulanase with that encoded by ywtF increased extracellular pullulanase activity by an additional 12%. Three-liter fermenter scale-up production yielded the highest extracellular pullulanase activity reported to date: 8037.91 U·mL−1. This study highlights the usefulness of dltB deletion and signal peptide optimization in enhancing extracellular protein production.





Similar content being viewed by others
Data Availability
Not applicable.
References
Westers, L., Westers, H., & Quax, W. J. (2004). Bacillus subtilis as cell factory for pharmaceutical proteins: A biotechnological approach to optimize the host organism. Biochimica et Biophysica Acta-Molecular Cell Research, 1694(1–3), 299–310.
Schallmey, M., Singh, A., & Ward, O. P. (2004). Developments in the use of Bacillus species for industrial production. Canadian Journal of Microbiology, 50(1), 1–17.
Li, W., Zhou, X., & Lu, P. (2004). Bottlenecks in the expression and secretion of heterologous proteins in Bacillus subtilis. Research in Microbiology, 155(8), 605–610.
Harwood, C. R., & Cranenburgh, R. (2008). Bacillus protein secretion: An unfolding story. Trends in Microbiology, 16(2), 73–79.
Caspers, M., Brockmeier, U., Degering, C., Eggert, T., & Freudl, R. (2010). Improvement of Sec-dependent secretion of a heterologous model protein in Bacillus subtilis by saturation mutagenesis of the N-domain of the AmyE signal peptide. Applied Microbiology and Biotechnology, 86(6), 1877–1885.
Westers, H., Westers, L., Darmon, E., van Dijl, J. M., Quax, W. J., & Zanen, G. (2006). The CssRS two-component regulatory system controls a general secretion stress response in Bacillus subtilis. FEBS Journal, 273(16), 3816–3827.
Sarvas, M., Harwood, C. R., Bron, S., & van Dijl, J. M. (2004). Post-translocational folding of secretory proteins in Gram-positive bacteria. Biochimica et Biophysica Acta-Molecular Cell Research, 1694(1–3), 311–327.
Beveridge, T. J., & Murray, R. G. (1980). Sites of metal deposition in the cell wall of Bacillus subtilis. Journal of Bacteriology, 141(2), 876–887.
Perego, M., Glaser, P., Minutello, A., Strauch, M. A., Leopold, K., & Fischer, W. (1995). Incorporation of D-alanine into lipoteichoic acid and wall teichoic acid in Bacillus subtilis. Identification of genes and regulation. Journal of Biological Chemistry, 270(26), 15598–15606.
Hyyrylainen, H. L., Vitikainen, M., Thwaite, J., Wu, H., Sarvas, M., Harwood, C. R., Kontinen, V. P., & Stephenson, K. (2000). D-Alanine substitution of teichoic acids as a modulator of protein folding and stability at the cytoplasmic membrane/cell wall interface of Bacillus subtilis. Journal of Biological Chemistry, 275(35), 26696–26703.
Vitikainen, M., Hyyrylainen, H. L., Kivimaki, A., Kontinen, V. P., & Sarvas, M. (2005). Secretion of heterologous proteins in Bacillus subtilis can be improved by engineering cell components affecting post-translocational protein folding and degradation. Journal of Applied Microbiology, 99(2), 363–375.
Thwaite, J. E., Baillie, L. W., Carter, N. M., Stephenson, K., Rees, M., Harwood, C. R., & Emmerson, P. T. (2002). Optimization of the cell wall microenvironment allows increased production of recombinant Bacillus anthracis protective antigen from B. subtilis. Applied and Environmental Microbiology, 68(1), 227–234.
Brockmeier, U., Caspers, M., Freudl, R., Jockwer, A., Noll, T., & Eggert, T. (2006). Systematic screening of all signal peptides from Bacillus subtilis: A powerful strategy in optimizing heterologous protein secretion in Gram-positive bacteria. Journal of Molecular Biology, 362(3), 393–402.
Degering, C., Eggert, T., Puls, M., Bongaerts, J., Evers, S., Maurer, K. H., & Jaeger, K. E. (2010). Optimization of protease secretion in Bacillus subtilis and Bacillus licheniformis by screening of homologous and heterologous signal peptides. Applied Microbiology and Biotechnology, 76(19), 6370–6376.
Yao, D., Su, L., Li, N., & Wu, J. (2019). Enhanced extracellular expression of Bacillus stearothermophilus α-amylase in Bacillus subtilis through signal peptide optimization, chaperone overexpression and α-amylase mutant selection. Microbial Cell Factories, 18, 69.
Fu, G., Liu, J., Li, J., Zhu, B., & Zhang, D. (2018). Systematic screening of optimal signal peptides for secretory production of heterologous proteins in Bacillus subtilis. Journal of Agricultural and Food Chemistry, 66(50), 13141–13151.
Zhang, K., Su, L., & Wu, J. (2018). Enhanced extracellular pullulanase production in Bacillus subtilis using protease-deficient strains and optimal feeding. Applied Microbiology and Biotechnology, 102(12), 5089–5103.
Zhang, K., Su, L., Duan, X., Liu, L., & Wu, J. (2017). High-level extracellular protein production in Bacillus subtilis using an optimized dual-promoter expression system. Microbial Cell Factories, 16, 32.
Anagnostopoulos, C., & Spizizen, J. (1961). Requirements for transformation in Bacillus subtilis. Journal of Bacteriology, 81(5), 741–746.
Zhang, K., Duan, X., & Wu, J. (2016). Multigene disruption in undomesticated Bacillus subtilis ATCC 6051a using the CRISPR/Cas9 system. Scientific Reports, 6, 27943.
Dempsey, L. A., & Dubnau, D. A. (1989). Localization of the replication origin of plasmid pE194. Journal of Bacteriology, 171(5), 2866–2869.
Bertoldo, C., Duffner, F., Jorgensen, P. L., & Antranikian, G. (1999). Pullulanase type I from Fervidobacterium pennavorans Ven5: Cloning, sequencing, and expression of the gene and biochemical characterization of the recombinant enzyme. Applied and Environmental Microbiology, 65(5), 2084–2091.
Lejeune, A., Sakaguchi, K., & Imanaka, T. (1989). A spectrophotometric assay for the cyclization activity of cyclomaltohexaose (α-cyclodextrin) glucanotransferase. Analytical Biochemistry, 181(1), 6–11.
Konsoula, Z., & Liakopoulou-Kyriakides, M. (2007). Co-production of alpha-amylase and β-galactosidase by Bacillus subtilis in complex organic substrates. Bioresource Technology, 98(1), 150–157.
Leloup, L., el Haddaoui, A., Chambert, R., & Petit-Glatron, M. F. (1997). Characterization of the rate-limiting step of the secretion of Bacillus subtilis α-amylase overproduced during the exponential phase of growth. Microbiology-SGM, 143(10), 3295–3303.
Funding
This work received financial support from the National Key Research and Development Program of China (2019YFA0706900), the National Natural Science Foundation of China (31730067, 31901633), the 111 Project (No. 111–2-06), the National First-Class Discipline Program of Light Industry Technology and Engineering (LITE2018–03), the Natural Science Foundation of Jiangsu Province (BK20180082), and the Jiangnan University Postdoctoral Science Foundation (5818088201191160).
Author information
Authors and Affiliations
Contributions
K Zhang and LQ Su designed the study. K Zhang performed the experiment. K Zhang, LQ Su, and J Wu analyzed the data. K Zhang wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, K., Su, L. & Wu, J. Enhancing Extracellular Pullulanase Production in Bacillus subtilis Through dltB Disruption and Signal Peptide Optimization. Appl Biochem Biotechnol 194, 1206–1220 (2022). https://doi.org/10.1007/s12010-021-03617-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-021-03617-6