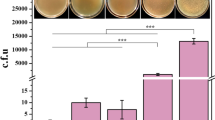
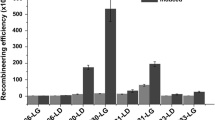
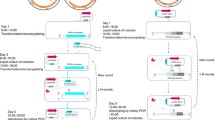
Abstract
Despite the great potential of Serratia marcescens in industrial applications, lack of powerful genetic modification tools limits understanding of the regulatory networks of the useful metabolites and therefore restricts their mass production. To meet the urgent demand, we established a genome-editing strategy for S. marcescens based on Red recombineering in this study. Without host modification in advance, nucA and pigA were substituted by PCR-amplified resistance genes. No long homologous arms were required at the two sides of resistance genes. Using this procedure, the fragment at the S. marcescens as large as 20 kb was easily deleted. Then we constructed a counter-selection gene kil constructed under the control of inducible PBAD operon, which demonstrates obvious lethality to S. marcescens. Subsequently, GmR-kil double selection cassette was inserted into the CDS of pigA gene. Using single-stranded DNA–mediated recombination, this insertion mutation was efficiently repaired through kil counter-selection. A powerful genetic modification platform based on Red recombineering system was successfully established for S. marcescens. Multiple types of modification and multiple recombination strategies can all be performed easily in this species. We hope this study will be useful for the theoretical research and the research of metabolic engineering in S. marcescens.





Similar content being viewed by others
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
References
Abreo, E., & Altier, N. (2019). Pangenome of Serratia marcescens strains from nosocomial and environmental origins reveals different populations and the links between them. Scientific Reports, 9(46), 1–8.
Yip, C. H., Yarkoni, O., Ajioka, J., Wan, K. L., & Nathan, S. (2019). Recent advancements in high-level synthesis of the promising clinical drug, prodigiosin. Applied Microbiology and Biotechnology, 103(4), 1667–1680.
Pan, X., Sun, C., Tang, M., You, J., Osire, T., Zhao, Y., Xu, M., Zhang, X., Shao, M., Yang, S., Yang, T., & Rao, Z. (2020). LysR-Type transcriptional regulator metr controls prodigiosin production, methionine biosynthesis, cell motility, h2o2 tolerance, heat tolerance, and exopolysaccharide synthesis in Serratia marcescens. Applied and Environmental Microbiology, 86(4), 1–18.
Emruzi, Z., Aminzadeh, S., Karkhane, A. A., Alikhajeh, J., Haghbeen, K., & Gholami, D. (2018). Improving the thermostability of Serratia marcescens B4A chitinase via G191V site-directed mutagenesis. International Journal of Biological Macromolecules, 116(2018), 64–70.
Visootsat, A., Nakamura, A., Vignon, P., Watanabe, H., Uchihashi, T., & Iino, R. (2020). Single-molecule imaging analysis reveals the mechanism of a high-catalytic-activity mutant of chitinase A from Serratia marcescens. Journal of Biological Chemistry, 295(7), 1915–1925.
Gerc, A. J., Song, L., Challis, G. L., Stanley-Wall, N. R., & Coulthurst, S. J. (2012). The insect pathogen Serratia marcescens Db10 uses a hybrid non-ribosomal peptide synthetase-polyketide synthase to produce the antibiotic althiomycin. PLoS One, 7(9), 1–13.
Velez-Gomez, J. M., Melchor-Moncada, J. J., Veloza, L. A., & Sepulveda-Arias, J. C. (2019). Purification and characterization of a metalloprotease produced by the C8 isolate of Serratia marcescens using silkworm pupae or casein as a protein source. International Journal of Biological Macromolecules, 135(2019), 97–105.
Ramesh, C., Vinithkumar, N. V., Kirubagaran, R., Venil, C. K., & Dufosse, L. (2019). Multifaceted applications of microbial pigments: current knowledge, challenges and future directions for public health implications. Microorganisms, 7(7), 1–46.
Darshan, N., & Manonmani, H. K. (2015). Prodigiosin and its potential applications. Journal of Food Science and Technology, 52(9), 5393–5407.
Montaner, B., Navarro, S., Pique, M., Vilaseca, M., Martinell, M., Giralt, E., Gil, J., & Perez-Tomas, R. (2000). Prodigiosin from the supernatant of Serratia marcescens induces apoptosis in haematopoietic cancer cell lines. British Journal of Pharmacology, 131(3), 585–593.
Han, S. B., Kim, H. M., Kim, Y. H., Lee, C. W., Jang, E. S., Son, K. H., Kim, S. U., & Kim, Y. K. (1998). T-cell specific immunosuppression by prodigiosin isolated from Serratia marcescens. International Journal of Immunopharmacology, 20(1-3), 1–13.
Lapenda, J. C., Silva, P. A., Vicalvi, M. C., Sena, K. X., & Nascimento, S. C. (2015). Antimicrobial activity of prodigiosin isolated from Serratia marcescens UFPEDA 398. World Journal of Microbiology and Biotechnology, 31(2), 399–406.
Bennett, J. W., & Bentley, R. (2000). Seeing red: the story of prodigiosin. Advances in Applied Microbiology, 47(2000), 1–32.
Herrero, M., de Lorenzo, V., & Timmis, K. N. (1990). Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. Journal of Bacteriology, 172(11), 6557–6567.
Shanks, R. M., Stella, N. A., Kalivoda, E. J., Doe, M. R., O’Dee, D. M., Lathrop, K. L., Guo, F. L., & Nau, G. J. (2007). A Serratia marcescens OxyR homolog mediates surface attachment and biofilm formation. Journal of Bacteriology, 189(20), 7262–7272.
Williamson, N. R., Simonsen, H. T., Ahmed, R. A., Goldet, G., Slater, H., Woodley, L., Leeper, F. J., & Salmond, G. P. (2005). Biosynthesis of the red antibiotic, prodigiosin, in Serratia: identification of a novel 2-methyl-3-n-amyl-pyrrole (MAP) assembly pathway, definition of the terminal condensing enzyme, and implications for undecylprodigiosin biosynthesis in Streptomyces. Molecular Microbiology, 56(4), 971–989.
Xu, P., Rizzoni, E. A., Sul, S. Y., & Stephanopoulos, G. (2017). Improving metabolic pathway efficiency by statistical model-based multivariate regulatory metabolic engineering. ACS Synthetic Biology, 6(1), 148–158.
Erb, T. J., Jones, P. R., & Bar-Even, A. (2017). Synthetic metabolism: metabolic engineering meets enzyme design. Current Opinion in Chemical Biology, 37(2017), 56–62.
Sharan, S. K., Thomason, L. C., Kuznetsov, S. G., & Court, D. L. (2009). Recombineering: A homologous recombination-based method of genetic engineering. Nature Protocols, 4(2), 206–223.
Yu, D., Ellis, H. M., Lee, E.-C., Jenkins, N. A., & Copeland, N. G. (2000). An efficient recombination system for chromosome engineering in Escherichia coli. Proceedings of the National Academy of Sciences, 97(11), 5978–5983.
Ellis, H. M., Yu, D., & DiTizio, T. (2001). High efficiency mutagenesis, repair, and engineering of chromosomal DNA using single-stranded oligonucleotides. Proceedings of the National Academy of Sciences, 98(12), 6742–6746.
Lee, E.-C., Yu, D., De Velasco, J. M., Tessarollo, L., Swing, D. A., Court, D. L., Jenkins, N. A., & Copeland, N. G. (2001). A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics, 73(1), 56–65.
Karlinsey, J. E. (2007). lambda-Red genetic engineering in Salmonella enterica serovar Typhimurium. Methods in Enzymology, 421(2007), 199–209.
Cox, M. M., Layton, S. L., Jiang, T., Cole, K., Hargis, B. M., Berghman, L. R., Bottje, W. G., & Kwon, Y. M. (2007). Scarless and site-directed mutagenesis in Salmonella enteritidis chromosome. BMC Biotechnology, 7(2007), 1–10.
Grin, I., Hartmann, M. D., Sauer, G., Hernandez Alvarez, B., Schutz, M., Wagner, S., Madlung, J., Macek, B., Felipe-Lopez, A., Hensel, M., Lupas, A., & Linke, D. (2014). A trimeric lipoprotein assists in trimeric autotransporter biogenesis in enterobacteria. Journal of Biological Chemistry, 289(11), 7388–7398.
Rossi, M. S., Paquelin, A., Ghigo, J. M., & Wandersman, C. (2003). Haemophore-mediated signal transduction across the bacterial cell envelope in Serratia marcescens: the inducer and the transported substrate are different molecules. Molecular Microbiology, 48(6), 1467–1480.
Kamaletdinova, L. K., Nizamutdinova, E. K., Shirshikova, T. V., Skipina, I. M., & Bogomolnaya, L. M. (2016). Inactivation of chromosomal genes in Serratia marcescens. Journal of Bionanoscience, 6(4), 376–378.
Chen, W., Li, Y., Wu, G., Zhao, L., Lu, L., Wang, P., Zhou, J., Cao, C., & Li, S. (2019). Simple and efficient genome recombineering using kil counter-selection in Escherichia coli. Journal of Biotechnology, 294(2019), 58–66.
Haeusser, D. P., Hoashi, M., Weaver, A., Brown, N., Pan, J., Sawitzke, J. A., Thomason, L. C., & Margolin, W. (2014). The Kil peptide of bacteriophage λ blocks Escherichia coli cytokinesis via ZipA-dependent inhibition of FtsZ assembly. PLoS Genetics, 10(3), 1–25.
Hornsey, M., Ellington, M. J., Doumith, M., Hudson, S., Livermore, D. M., & Woodford, N. (2010). Tigecycline resistance in Serratia marcescens associated with up-regulation of the SdeXY-HasF efflux system also active against ciprofloxacin and cefpirome. Journal of Antimicrobial Chemotherapy, 65(3), 479–482.
Fuste, E., Galisteo, G. J., Jover, L., Vinuesa, T., Villa, T. G., & Vinas, M. (2012). Comparison of antibiotic susceptibility of old and current Serratia. Future Microbiology, 7(6), 781–786.
Shokouhfard, M., Kermanshahi, R. K., Shahandashti, R. V., Feizabadi, M. M., & Teimourian, S. (2015). The inhibitory effect of a Lactobacillus acidophilus derived biosurfactant on biofilm producer Serratia marcescens. Iranian Journal of Basic Medical Sciences, 18(10), 1001–1007.
Guzman, L. M., Belin, D., Carson, M. J., & Beckwith, J. (1995). Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. Journal of Bacteriology, 177(14), 4121–4130.
Huang, Y. W., Hu, R. M., Chiang, Y. T., Chung, T. C., Chung, T. C., & Yang, T. C. (2011). Establishment of an arabinose-inducible system in Stenotrophomonas maltophilia. Folia Microbiologica, 56(1), 18–22.
Okrent, R. A., Trippe, K. M., Maselko, M., & Manning, V. (2017). Functional analysis of a biosynthetic cluster essential for production of 4-formylaminooxyvinylglycine, a germination-arrest factor from Pseudomonas fluorescens WH6. Microbiology (Reading), 163(2), 207–217.
Hu, D. X., Withall, D. M., Challis, G. L., & Thomson, R. J. (2016). Structure, chemical synthesis, and biosynthesis of prodiginine natural products. Chemical Reviews, 116(14), 7818–7853.
Soo, P. C., Horng, Y. T., Chang, Y. L., Tsai, W. W., Jeng, W. Y., Lu, C. C., & Lai, H. C. (2014). ManA is regulated by RssAB signaling and promotes motility in Serratia marcescens. Research in Microbiology, 165(1), 21–29.
Romanowski, E. G., Lehner, C. M., Martin, N. C., Patel, K. R., Callaghan, J. D., Stella, N. A., & Shanks, R. M. Q. (2019). Thermoregulation of prodigiosin biosynthesis by Serratia marcescens is controlled at the transcriptional level and requires HexS. Polish Journal of Microbiology, 68(1), 43–50.
Haddix, P. L., & Shanks, R. M. Q. (2020). Production of prodigiosin pigment by Serratia marcescens is negatively associated with cellular ATP levels during high-rate, low-cell-density growth. Canadian Journal of Microbiology, 66(3), 243–255.
Acknowledgements
Special thanks to Professor Donald L. Court for the plasmid (pKD46 and pSim6) and Sheng Yang for the strain MG1655.
Funding
This study was supported by the Science and Technology Program of Guangdong Province (grant number 2015A010107014) and Grant-in-Aid from the Natural Scientific Foundation of China (grant number 31501895).
Author information
Authors and Affiliations
Contributions
W Chen and RY Chen performed the research; W Chen designed the experiments and wrote the manuscript. JY Cao supervised the research and helped to draft the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All authors have read and approved this version of the article and consented for publication.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
• PCR-amplified products with short homology were used for recombination.
• A usable counter-selection system based on kil gene was constructed.
• Insertion mutation can be repaired efficiently with ssDNA-meditated recombination.
Rights and permissions
About this article
Cite this article
Chen, W., Chen, R. & Cao, J. Rapid Genome Modification in Serratia marcescens Through Red Homologous Recombination. Appl Biochem Biotechnol 193, 2916–2931 (2021). https://doi.org/10.1007/s12010-021-03576-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-021-03576-y