Abstract
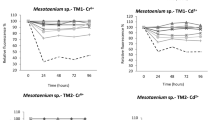
Ionic liquids are widely used for lipid and pigment extractions from microalgae. It is possible that ionic liquids are discharged into environments. The evaluation of growth performance and antioxidative response of ionic liquids to microalgae is helpful to explore the stress regulation mechanism and investigate possible environmental risk. Ionic liquids induce production of reactive oxygen species (ROS) to microalgae. These oxidative stresses are possible from cations, anions, and salinity. In this study, the growth inhibitions of [BMIM]Br, [BMIM]Cl, [EMIM]Cl, and [EMIM]EtOSO3 to Anabaena cylindrica, Chlorella pyrenoidesa, and Dunaliella salina were evaluated. It was interesting that Br− and two kinds of cations, [BMIM] and [EMIM], had significant effects on growth inhibitions of these microalgae. IC50 values of these ionic liquids for A. cylindrica, C. pyrenoidesa, and D. salina were also estimated based on the results of growth inhibitions. It was proved that [EMIM]Cl is relatively harmless to C. pyrenoidesa and D. salina, and [EMIM]EtOSO3 is relatively or practically harmless to C. pyrenoidesa. [BMIM]Br and [BMIM]Cl are practically harmless to A. cylindrica and C. pyrenoidesa, and relatively harmless to D. salina. More than 0.8 g/L [EMIM]EtOSO3 led to bleaching of both A. cylindrica and D. salina at 48 h which was shown that the anion, EtOSO3−, had higher inhibition to A. cylindrica and D. salina than Cl−. In addition, high concentration of ionic liquids led to reductions of chlorophyll content in these three kinds of microalgae, increase of ROS levels and malondialdehyde contents for most of the cases. High concentration of ionic liquids also increased the activities of superoxide dismutase in three kinds of microalgae. There were positive correlations between ROS levels or MDA content, and inhibitions ratios of these ionic liquids to microalgae except [EMIM]Cl to A. cylindrica. These antioxidant enzymes were beneficial for reducing the ROS induced by ionic liquids.








Similar content being viewed by others
Data Availability
Not available.
References
Kumar, J. S. P., Garlapati, V. K., Dash, A., Scholz, P., & Banerjee, R. (2017). Sustainable green solvents and techniques for lipid extraction from microalgae: a review. Algal Research, 21, 138–147.
Itoh, T. (2017). Ionic liquids as tool to improve enzymatic organic synthesis. Chemical Reviews, 117(15), 10567–10607.
Choi, S.-A., Lee, J.-S., Oh, Y.-K., Jeong, M.-J., Kim, S., & Park, J.-Y. (2014). Lipid extraction from Chlorella vulgaris by molten-salt/ionic-liquid mixtures. Algal Research, 3, 44–48.
Lu, H., Yu, X., Li, H., Tu, S.-T., & Sebastian, S. (2019). Lipids extraction from wet Chlorella pyrenoidosa sludge using recycled BMIM Cl. Bioresource Technology, 291, 121819.
Desai, R. K., Streefland, M., Wijffels, R. H., & Eppink, M. H. M. (2016). Novel astaxanthin extraction from Haematococcus pluvialis using cell permeabilising ionic liquids. Green Chemistry, 18(5), 1261–1267.
Zeng, H., Wang, Y., Kong, J., Nie, C., & Yuan, Y. (2010). Ionic liquid-based microwave-assisted extraction of rutin from Chinese medicinal plants. Talanta, 83(2), 582–590.
Abbas, M., Adil, M., Ehtisham-ul-Haque, S., Munir, B., Yameen, M., Ghaffar, A., Shar, G. A., Tahir, M. A., & Iqbal, M. (2018). Vibrio fischeri bioluminescence inhibition assay for ecotoxicity assessment: a review. Science of the Total Environment, 626, 1295–1309.
Biczak, R., Pawlowska, B., Balczewski, P., & Rychter, P. (2014). The role of the anion in the toxicity of imidazolium ionic liquids. Journal of Hazardous Materials, 274, 181–190.
Cheng, D., Li, X., Yuan, Y., Yang, C., Tang, T., Zhao, Q., & Sun, Y. (2019). Adaptive evolution and carbon dioxide fixation of Chlorella sp. in simulated flue gas. Science of the Total Environment, 650, 2931–2938.
Li, D., Wang, L., Zhao, Q., Wei, W., & Sun, Y. (2015). Improving high carbon dioxide tolerance and carbon dioxide fixation capability of Chlorella sp. by adaptive laboratory evolution. Bioresource Technology, 185, 269–275.
Wang, L., Xue, C., Wang, L., Zhao, Q., Wei, W., & Sun, Y. (2016). Strain improvement of Chlorella sp. for phenol biodegradation by adaptive laboratory evolution. Bioresource Technology, 205, 264–268.
Li, D., Yuan, Y., Cheng, D., & Zhao, Q. (2019). Effect of light quality on growth rate, carbohydrate accumulation, fatty acid profile and lutein biosynthesis of Chlorella sp. AE10. Bioresource Technology, 291, 121783.
t Lam, G. P., Vermue, M. H., Eppink, M. H. M., Wijffels, R. H., & van den Berg, C. (2018). Multi-product microalgae biorefineries: from concept towards reality. Trends in Biotechnology, 36, 216–227.
Li, H., Zhao, Q., & Huang, H. (2019). Current states and challenges of salt-affected soil remediation by cyanobacteria. Science of the Total Environment, 669, 258–272.
Costa, S. P. F., Pinto, P. C. A. G., Saraiva, M. L. M. F. S., Rocha, F. R. P., Santos, J. R. P., & Monteiro, R. T. R. (2015). The aquatic impact of ionic liquids on freshwater organisms. Chemosphere, 139, 288–294.
Pawlowska, B., Telesinski, A., & Biczak, R. (2019). Phytotoxicity of ionic liquids. Chemosphere, 237, 124436–124436.
Deng, X.-Y., Hu, X.-L., Cheng, J., Ma, Z.-X., & Gao, K. (2016). Growth inhibition and oxidative stress induced by 1-octyl-3-methylimidazolium bromide on the marine diatom Skeletonema costatum. Ecotoxicology and Environmental Safety, 132, 170–177.
Sena, D. W., Kulacki, K. J., Chaloner, D. T., & Lamberti, G. A. (2010). The role of the cell wall in the toxicity of ionic liquids to the alga Chlamydomonas reinhardtii. Green Chemistry, 12(6), 1066–1071.
Latala, A., Nedzi, M., & Stepnowski, P. (2009). Toxicity of imidazolium and pyridinium based ionic liquids towards algae. Chlorella vulgaris, Oocystis submarina (green algae) and Cyclotella meneghiniana, Skeletonema marinoi (diatoms). Green Chemistry, 11(4), 580–588.
Zhang, C., Zhang, S., Zhu, L., Wang, J., Wang, J., & Zhou, T. (2017). The acute toxic effects of 1-alkyl-3-methylimidazolium nitrate ionic liquids on Chlorella vulgaris and Daphnia magna. Environmental Pollution, 229, 887–895.
Araujo, O. Q. F., Gobbi, C. N., Chaloub, R. M., & Coelho, M. A. Z. (2009). Assessment of the impact of salinity and irradiance on the combined carbon dioxide sequestration and carotenoids production by Dunaliella salina: A mathematical model. Biotechnology and Bioengineering, 102(2), 425–435.
Xue, C., Wang, L., Wu, T., Zhang, S., Tang, T., Wang, L., Zhao, Q., & Sun, Y. (2017). Characterization of co-cultivation of cyanobacteria on growth, productions of polysaccharides and extracellular proteins, nitrogenase activity, and photosynthetic activity. Applied Biochemistry and Biotechnology, 181(1), 340–349.
Stolte, S., Matzke, M., Arning, J., Boeschen, A., Pitner, W.-R., Welz-Biermann, U., Jastorff, B., & Ranke, J. (2007). Effects of different head groups and functionalised side chains on the aquatic toxicity of ionic liquids. Green Chemistry, 9(11), 1170–1179.
Xia, Y., Liu, D., Dong, Y., Chen, J., & Liu, H. (2018). Effect of ionic liquids with different cations and anions on photosystem and cell structure of Scenedesmus obliquus. Chemosphere, 195, 437–447.
Wahidin, S., Idris, A., & Shaleh, S. R. M. (2016). Ionic liquid as a promising biobased green solvent in combination with microwave irradiation for direct biodiesel production. Bioresource Technology, 206, 150–154.
Tsarpali, V., & Dailianis, S. (2015). Toxicity of two imidazolium ionic liquids, bmim BF4 and omim BF4 , to standard aquatic test organisms: Role of acetone in the induced toxicity. Ecotoxicology and Environmental Safety, 117, 62–71.
Tsarpali, V., Harbi, K., & Dailianis, S. (2016). Physiological response of the green microalgae Dunaliella tertiolecta against imidazolium ionic liquids bmim BF4 and/or omim BF4 : the role of salinity on the observed effects. Journal of Applied Phycology, 28(2), 979–990.
Fan, H., Jin, M., Wang, H., Xu, Q., Xu, L., Wang, C., Du, S., & Liu, H. (2019). Effect of differently methyl-substituted ionic liquids on Scenedesmus obliquus growth, photosynthesis, respiration, and ultrastructure. Environmental Pollution, 250, 155–165.
Latala, A., Nedzi, M., & Stepnowski, P. (2010). Toxicity of imidazolium ionic liquids towards algae. Influence of salinity variations. Green Chemistry, 12(1), 60–64.
Cho, C.-W., Pham, T. P. T., Jeon, Y.-C., Vijayaraghavan, K., Choe, W.-S., & Yun, Y.-S. (2007). Toxicity of imidazolium salt with anion bromide to a phytoplankton Selenastrum capricornutum: effect of alkyl-chain length. Chemosphere, 69(6), 1003–1007.
Ma, J.-M., Cai, L.-L., Zhang, B.-J., Hu, L.-W., Li, X.-Y., & Wang, J.-J. (2010). Acute toxicity and effects of 1-alkyl-3-methylimidazolium bromide ionic liquids on green algae. Ecotoxicology and Environmental Safety, 73(6), 1465–1469.
Chen, H., Zou, Y., Zhang, L., Wen, Y., & Liu, W. (2014). Enantioselective toxicities of chiral ionic liquids 1-alkyl-3-methylimidazolium lactate to aquatic algae. Aquatic Toxicology, 154, 114–120.
Bauer, T., Hager, V., Williams, M. B., Laurin, M., Doepper, T., Goerling, A., Szesni, N., Wasserscheid, P., Haumann, M., & Libuda, J. (2017). Palladium-mediated ethylation of the imidazolium cation monitored in operando on a solid catalyst with ionic liquid layer. Chemcatchem, 9(1), 109–113.
Nemestothy, N., Megyeri, G., Bakonyi, P., Lakatos, P., Kook, L., Polakovic, M., Gubicza, L., & Belafi-Bako, K. (2017). Enzyme kinetics approach to assess biocatalyst inhibition and deactivation caused by [bmim][Cl] ionic liquid during cellulose hydrolysis. Bioresource Technology, 229, 190–195.
Zhou, L., Yuan, Y., Li, X., Mei, S., Gao, J., Zhao, Q., Wei, W., & Sun, Y. (2018). Exploration of phenol tolerance mechanism through antioxidative responses of an evolved strain, Chlorella sp. L5. Journal of Applied Phycology, 30(4), 2379–2385.
Rezayian, M., Niknam, V., & Faramarzi, M. A. (2019). Antioxidative responses of Nostoc ellipsosporum and Nostoc piscinale to salt stress. Journal of Applied Phycology, 31(1), 157–169.
Saeki, K., Aburai, N., Aratani, S., Miyashita, H., & Abe, K. (2017). Salt-stress and plant hormone-like responses for selective reactions of esterified xanthophylls in the aerial microalga Coelastrella sp KGU-Y002. Journal of Applied Phycology, 29(1), 115–122.
Verma, E., Chakraborty, S., Tiwari, B., Singh, S., & Mishra, A. K. (2018). Alleviation of NaCl toxicity in the cyanobacterium Synechococcus sp PCC 7942 by exogenous calcium supplementation. Journal of Applied Phycology, 30(3), 1465–1482.
Moghimifam, R., Niknam, V., Ebrahimzadeh, H., & Hejazi, M. A. (2020). The influence of different CO2 concentrations on the biochemical and molecular response of two isolates of Dunaliella sp. (ABRIINW-CH2 and ABRIINW-SH33). Journal of Applied Phycology, 32(1), 175–187.
Jin, M., Wang, H., Li, Z., Fu, L., Chu, L., Wu, J., Du, S., & Liu, H. (2019). Physiological responses of Chlorella pyrenoidosa to 1-hexyl-3-methyl chloride ionic liquids with different cations. Science of the Total Environment, 685, 315–323.
Kim, G.-Y., Heo, J., Kim, H.-S., & Han, J.-I. (2017). Bicarbonate-based cultivation of Dunaliella salina for enhancing carbon utilization efficiency. Bioresource Technology, 237, 72–77.
Yoon, J. H., Choi, S. S., & Park, T. H. (2012). The cultivation of Anabaena variabilis in a bubble column operating under bubbly and slug flows. Bioresource Technology, 110, 430–436.
Sebaugh, J. L. (2011). Guidelines for accurate EC50/IC50 estimation. Pharmaceutical Statistics, 10(2), 128–134.
Li, J.-L., Liu, X.-Y., Xie, J.-T., Di, Y.-L., & Zhu, F.-X. (2015). A comparison of different estimation methods for fungicide EC50 and EC95 values. Journal of Phytopathology, 163(4), 239–244.
Liu, H., Zhang, X., Chen, C., Du, S., & Dong, Y. (2015). Effects of imidazolium chloride ionic liquids and their toxicity to Scenedesmus obliquus. Ecotoxicology and Environmental Safety, 122, 83–90.
Pruvost, J., Van Vooren, G., Le Gouic, B., Couzinet-Mossion, A., & Legrand, J. (2011). Systematic investigation of biomass and lipid productivity by microalgae in photobioreactors for biodiesel application. Bioresource Technology, 102(1), 150–158.
Deamici, K. M., Cuellar-Bermudez, S. P., Muylaert, K., Santos, L. O., & Costa, J. A. V. (2019). Quantum yield alterations due to the static magnetic fields action on Arthrospira platensis SAG 21.99: evaluation of photosystem activity. Bioresource Technology, 292, 121945.
Liu, D., Liu, H., Wang, S., Chen, J., & Xia, Y. (2018). The toxicity of ionic liquid 1-decylpyridinium bromide to the algae Scenedesmus obliquus: growth inhibition, phototoxicity, and oxidative stress. Science of the Total Environment, 622, 1572–1580.
Chen, B., Xue, C., Amoah, P. K., Li, D., Gao, K., & Deng, X. (2019). Impacts of four ionic liquids exposure on a marine diatom Phaeodactylum tricornutum at physiological and biochemical levels. Science of the Total Environment, 665, 492–501.
Fan, H., Liu, H., Dong, Y., Chen, C., Wang, Z., Guo, J., & Du, S. (2019). Growth inhibition and oxidative stress caused by four ionic liquids in Scenedesmus obliquus: role of cations and anions. Science of the Total Environment, 651, 570–579.
Xiong, J.-Q., Kurade, M. B., Kim, J. R., Roh, H.-S., & Jeon, B.-H. (2017). Ciprofloxacin toxicity and its co-metabolic removal by a freshwater microalga Chlamydomonas mexicana. Journal of Hazardous Materials, 323, 212–219.
Passino, D. R. M., & Smith, S. B. (1987). Acute bioassays and hazard evaluation of representative contaminants detected in great-lakes fish. Environmental Toxicology and Chemistry, 6(11), 901–907.
Cho, K., Lee, C.-H., Ko, K., Lee, Y.-J., Kim, K.-N., Kim, M.-K., Chung, Y.-H., Kim, D., Yeo, I.-K., & Oda, T. (2016). Use of phenol-induced oxidative stress acclimation to stimulate cell growth and biodiesel production by the oceanic microalga Dunaliella salina. Algal Research, 17, 61–66.
Pham, T. P. T., Cho, C.-W., Min, J., & Yun, Y.-S. (2008). Alkyl-chain length effects of imidazolium and pyridinium ionic liquids on photosynthetic response of Pseudokirchneriella subcapitata. Journal of Bioscience and Bioengineering, 105(4), 425–428.
Deng, X. Y., Li, D., Wang, L., Hu, X. L., Cheng, J., & Gao, K. (2017). Potential toxicity of ionic liquid ([C12mim]BF4) on the growth and biochemical characteristics of a marine diatom Phaeodactylum tricornutum. Science of the Total Environment, 586, 675–684.
Grudzinski, W., Krzeminska, I., Luchowski, R., Nosalewicz, A., & Gruszecki, W. I. (2016). Strong-light-induced yellowing of green microalgae Chlorella: a study on molecular mechanisms of the acclimation response. Algal Research, 16, 245–254.
Liu, H., Wu, J., Zhang, X., Xia, Y., Li, Y., & Du, S. (2017). Enantioselective oxidative stress caused by chiral ionic liquids forms of 1-alkyl-3-methyl imidazolium tartrate on Scenedesmus obliquus. Science of the Total Environment, 595, 819–827.
Mittler, R. (2002). Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science, 7(9), 405–410.
Swapnil, P., Yadav, A. K., Srivastav, S., Sharma, N. K., Srikrishna, S., & Rai, A. K. (2017). Biphasic ROS accumulation and programmed cell death in a cyanobacterium exposed to salinity (NaCl and Na2SO4). Algal Research, 23, 88–95.
Funding
This study is supported by the National Natural Science Foundation of China (21576278).
Author information
Authors and Affiliations
Contributions
Y.L. Zhu: formal analysis, data curation, writing - original draft, writing - review and editing; X.Q. Zheng: formal analysis, writing - review and editing; Y.J. Wang: writing - review and editing; Q.Y. Zhao: conceptualization, formal analysis, writing - original draft, writing - review and editing, project administration; H. Huang: supervision, writing - review and editing.
Corresponding authors
Ethics declarations
Ethical Approval
The study is about microalgae culture and did not need ethical approval.
Consent to Participate
The study is about microalgae culture and no human is involved in it.
Consent to Publish
The study is about microalgae culture and no human is involved in it.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhu, Y., Zhong, X., Wang, Y. et al. Growth Performance and Antioxidative Response of Chlorella pyrenoidesa, Dunaliella salina, and Anabaena cylindrica to Four Kinds of Ionic Liquids. Appl Biochem Biotechnol 193, 1945–1966 (2021). https://doi.org/10.1007/s12010-021-03515-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-021-03515-x