Abstract
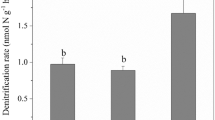
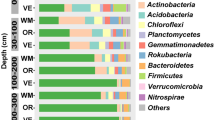
Studies have shown that the addition of biochar to agricultural soils has the potential to mitigate climate change by decreasing nitrous oxide (N2O) emissions resulting from denitrification. Rice paddy field soils have been known to have strong denitrifying activity, but the response of microbes to biochar for weakening denitrification in rice paddy field soils is not well known. In this work, compared with the chemical fertilizer alone, the chemical fertilizer + 20 t hm−2 biochar fertilizer slightly decreased denitrifying the nitrite reductase activity (S-NiR) and N2O emission without statistic difference, whereas the chemical fertilizer + 40 t hm−2 biochar significantly boosted them. The abundance of nir-denitrifiers contributed to S-NiR and N2O emission, especially nirS-denitrifiers, rather than the variation of community structure. Pearson correlation analysis showed that NO2−-N was a key factor for controlling the abundance of nir-denitrifiers, S-NiR and N2O emission. The biochar addition fertilization treatments strongly shaped the community structure of nirK-denitrifiers, while the community structure of nirS-denitrifiers remained relatively stable. In addition, Paracoccus and Sinorhizobium were revealed to be as the predominant lineage of nirS- and nirK-containing denitrifiers, respectively. Distance-based redundancy analysis (db-RDA) showed that changes in the nir-denitrifier community structure were significantly related to soil organic carbon, NO3−-N, and total phosphorus. Our findings suggest that, although the nirS- and nirK-denitrifiers are both controlling nitrite reductase, their responses to biochar addition fertilization treatments showed significant discrepancies of diversity, abundance, and contribution to N2O and S-NiR in a paddy soil.






Similar content being viewed by others
References
Ravishankara, A. R., Daniel, J. S., & Portmann, R. W. (2009). Nitrous oxide (n2o): the dominant ozone-depleting substance emitted in the 21st century. Science, 326(5949), 123–125.
WMO, Greenhouse Gas Bulletin. World Meterological Organization 2016. Available from: http://www.wmo.int/gaw/. Acessed June 26, 2018.
Butterbachbahl, K., Baggs, E. M., Dannenmann, M., Kiese, R., & Zechmeisterboltenstern, S. (2013). Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 368(1621), 20130122.
Kögel-Knabner, I., Amelung, W., Cao, Z. H., Fiedler, S., Frenzel, P., Jahn, R., et al. (2010). Biogeochemistry of paddy soils. Geoderma, 157(1), 1–14.
Azziz, G., Monza, J., Etchebehere, C., & Irisarri, P. (2017). Nirs- and nirk-type denitrifier communities are differentially affected by soil type, rice cultivar and water management. European Journal of Soil Biology, 78(Complete), 20–28.
Conrad, R. (2002). Control of microbial methane production in wetland rice fields. Nutrient Cycling in Agroecosystems, 64(1–2), 59–69.
Syakila, A., & Kroeze, C. (2011). The global nitrous oxide budget revisited. Greenhouse Gas Measurement & Management, 1(1), 17–26.
Ji, Y., Liu, G., Ma, J., Zhang, G., Xu, H., & Yagi, K. (2013). Effect of controlled-release fertilizer on mitigation of n2o emission from paddy field in South China: A multi-year field observation. Plant and Soil, 371(1–2), 473–486.
Krause, H. M., Hüppi, R., Leifeld, J., Elhadidi, M., Harter, J., Kappler, A., et al. (2018). Biochar affects community composition of nitrous oxide reducers in a field experiment. Soil Biology & Biochemistry, 119, 143–151.
Harter, J., El-Hadidi, M., Huson, D. H., Kappler, A., & Behrens, S. (2017). Soil biochar amendment affects the diversity of nosz transcripts: Implications for n2o formation. Scientific Reports, 7(1), 3338.
Yang, S., Xiao, Y., Sun, X., Ding, J., Jiang, Z., & Xu, J. (2019). Biochar improved rice yield and mitigated ch4 and n2o emissions from paddy field under controlled irrigation in the taihu lake region of China. Atmospheric Environment, 200, 69–77.
Atkinson, C. J., Fitzgerald, J. D., & Hipps, N. A. (2010). Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant and Soil, 337(1–2), 1–18.
Sohi, S. P. (2012). Agriculture. Carbon storage with benefits. Science, 338(6110), 1034–1035.
Cayuela, M. L., Sánchez-Monedero, M. A., Roig, A., Hanley, K., Enders, A., & Lehmann, J. (2013). Biochar and denitrification in soils: when, how much and why does biochar reduce n2o emissions? Scientific Reports, 3, 1732.
Harter, J., Krause, H. M., Schuettler, S., Ruser, R., Fromme, M., Scholten, T., et al. (2014). Linking n2o emissions from biochar-amended soil to the structure and function of the n-cycling microbial community. Isme Journal Multidisciplinary Journal of Microbial Ecology, X(X), 8(3), 660–674.
Van Zwieten, L., Singh, B. P., Kimber, S. W. L., Murphy, D. V., Macdonald, L. M., Rust, J., et al. (2014). An incubation study investigating the mechanisms that impact n2o flux from soil following biochar application. Agriculture, Ecosystems & Environment, 191, 53–62.
Baggs, E. M., Smales, C. L., & Bateman, E. J. (2010). Changing ph shifts the microbial sourceas well as the magnitude of n2o emission from soil. Biology and Fertility of Soils, 46(8), 793–805.
Cuhel, J., Simek, M., Laughlin, R. J., Bru, D., Cheneby, D., Watson, C. J., et al. (2010). Insights into the effect of soil ph on n2o and n2 emissions and denitrifier community size and activity. Applied and Environmental Microbiology, 76(6), 1870–1878.
Gul, S., & Whalen, J. K. (2016). Biochemical cycling of nitrogen and phosphorus in biochar-amended soils. Soil Biology & Biochemistry, 103, 1–15.
Clough, T., Condron, L., Kammann, C., & Müller, C. (2013). A review of biochar and soil nitrogen dynamics. Agronomy Journal, 3, 275–293.
Cayuela, M. L., Van Zwieten, L., Singh, B. P., Jeffery, S., Roig, A., & Sánchez-Monedero, M. A. (2014). Biochar’s role in mitigating soil nitrous oxide emissions: A review and meta-analysis. Agriculture, Ecosystems & Environment, 191, 5–16.
Seitzinger, S., Harrison, J. A., Böhlke, J. K., Bouwman, A. F., Lowrance, R., Peterson, B., et al. (2006). Denitrification across landscapes and waterscapes: A synthesis. Ecological Applications, 16(6), 2064–2090.
Braker, G., & Conrad, R. (2011). Diversity, structure, and size of n2o-producing microbial communities in soilsâ--“what matters for their functioning”? Advances in Applied Microbiology, 75, 33–70.
Cutruzzola, F., Brown, K., Wilson, E. K., Bellelli, A., Arese, M., Tegoni, M., et al. (2001). The nitrite reductase from pseudomonas aeruginosa: Essential role of two active-site histidines in the catalytic and structural properties. Proceedings of the National Academy of Sciences, 98(5), 2232–2237.
Zumft, W. G. (1997). Cell biology and molecular basis of denitrification. Microbiology and Molecular Biology Reviews, 61(4), 533–616.
Jones, C. M., & Hallin, S. (2010). Ecological and evolutionary factors underlying global and local assembly of denitrifier communities. ISME Journal, 4(5), 633–641.
Lee, J. A., & Francis, C. A. (2017). Spatiotemporal characterization of San Francisco bay denitrifying communities: A comparison ofnirkandnirsdiversity and abundance. Microbial Ecology, 73(2), 271–284.
Hou, S., Ai, C., Zhou, W., Liang, G., & He, P. (2018). Structure and assembly cues for rhizospheric, nirk - and, nirs -type denitrifier communities in long-term fertilized soils. Soil Biology and Biochemistry, 119, 32–40.
Yoshida, M., Ishii, S., Otsuka, S., & Senoo, K. (2009). Temporal shifts in diversity and quantity of nirs and nirk in a rice paddy field soil. Soil Biology & Biochemistry, 41(10), 2044–2051.
Yin, C., Fan, F., Song, A., Li, Z., Yu, W., & Liang, Y. (2014). Different denitrification potential of aquic brown soil in Northeast China under inorganic and organic fertilization accompanied by distinct changes of nirs- and nirk-denitrifying bacterial community. European Journal of Soil Biology, 65, 47–56.
Sanford, R. A., Wagner, D. D., Wu, Q., Chee-Sanford, J. C., Thomas, S. H., Cruz-Garcia, C., et al. (2012). Unexpected nondenitrifier nitrous oxide reductase gene diversity and abundance in soils. Proceedings of the National Academy of Sciences, 109(48), 19709–19714.
Dambreville, C., Hénault, C., Bizouard, F., Morvan, T., Chaussod, R., & Germon, J. C. (2006). Compared effects of long-term pig slurry applications and mineral fertilization on soil denitrification and its end products (n2o, n2). Biology and Fertility of Soils, 42(6), 490–500.
Huang, X. X., Gao, M., Wei, C. F., Xie, D. T., & Pan, G. X. (2006). Tillage effect on organic carbon in a purple paddy soil. Pedosphere, 16(5), 0–667.
Huang, R., Lan, M., Liu, J., & Gao, M. (2017). Soil aggregate and organic carbon distribution at dry land soil and paddy soil: The role of different straws returning. Environmental Science & Pollution Research, 24(36), 1–11.
Riya, S., Katayama, M., Takahashi, E., Zhou, S., & Terada, A. (2014). Mitigation of greenhouse gas emissions by water management in a forage rice paddy field supplemented with dry-thermophilic anaerobic digestion residue. Water, Air, & Soil Pollution, 225(9), 2118.
Hou, H., Peng, S., Xu, J., Yang, S., & Mao, Z. (2012). Seasonal variations of ch4 and n2o emissions in response to water management of paddy fields located in Southeast China. Chemosphere, 89(7), 884–892.
Bolan, N. S., Baskaran, S., & Thiagarajan, S. (1996). An evaluation of the methods of measurement of dissolved organic carbon in soils, manures, sludges, and stream water. Communications in Soil Science and Plant Analysis, 27(13–14), 2723–2737.
Lu, R. K. (2000). Methods of soil and A gro-chemical analysis. Beijing: China agricultural science and technology press.
Yang, J. H., Wang, C. L., & Dai, H. L. (2008). Agricultural soil analysis and environmental monitoring. Beijing: China Land Press (in Chinese).
Michotey, V., Méjean, V., & Bonin, P. (2000). Comparison of methods for quantification of cytochrome cd1-denitrifying bacteria in environmental marine samples. Applied and Environmental Microbiology, 66(4), 1564–1571.
Throback, I. N., Enwall, K., Jarvis, Å., & Hallin, S. (2004). Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. FEMS Microbiology Ecology, 49(3), 401–417.
Chen, Z., Luo, X., Hu, R., Wu, M., Wu, J., & Wei, W. (2010). Impact of long-term fertilization on the composition of denitrifier communities based on nitrite reductase analyses in a paddy soil. Microbial Ecology, 60(4), 850–861.
Schloss, P. D., Westcott, S. L., Ryabin, T., Hall, J. R., Hartmann, M., Hollister, E. B., Lesniewski, R. A., Oakley, B. B., Parks, D. H., Robinson, C. J., Sahl, J. W., Stres, B., Thallinger, G. G., van Horn, D., & Weber, C. F. (2009). Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Applied and Environmental Microbiology, 75(23), 7537–7541.
Henry, S., Baudoin, E., López-Gutiérrez, J. C., Martin-Laurent, F., Brauman, A., & Philippot, L. (2004). Quantification of denitrifying bacteria in soils by nirk, gene targeted real-time PCR. Journal of Microbiological Methods, 59(3), 327–335.
Culman, S. W., Bukowski, R., Gauch, H. G., Cadillo-Quiroz, H., & Buckley, D. H. (2009). T-rex: Software for the processing and analysis of t-rflp data. BMC Bioinformatics, 10(1), 171–170.
Atlas, R. M., & Bartha, R. (1987). Microbial ecology: Fundamentals and applications (2nd ed.). Menlo Park: Benjamin Cummings Publishing Co..
By Jan Lepš. (2003). Multivariate analysis of ecological data using CANOCO 5. Multivariate analysis of ecological data using CANOCO. Cambridge University Press.
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M., & Kumar, S. (2011). Mega5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 28(10), 2731–2739.
Krapfl, K. J., Hatten, J. A., Roberts, S. D., Baldwin, B. S., Rousseau, R. J., & Shankle, M. W. (2014). Soil properties, nitrogen status, and switchgrass productivity in a biochar-amended silty clay loam. Soil Science Society of America Journal, 78(S1), S136.
Streubel, J. D., Collins, H. P., Garcia-Perez, M., Tarara, J., Granatstein, D., & Kruger, C. E. (2011). Influence of contrasting biochar types on five soils at increasing rates of application. Soil Science Society of America Journal, 75(4), 1402.
Diehl, D., Bayer, J. V., Woche, S. K., Bryant, R., Doerr, S. H., & Schaumann, G. E. (2010). Reaction of soil water repellency to artificially induced changes in soil ph. Geoderma, 158(3–4), 0–384.
Huang, M., Zhou, X., Chen, J., Cao, F., Jiang, L., & Zou, Y. (2017). Interaction of changes in ph and urease activity induced by biochar addition affects ammonia volatilization on an acid paddy soil following application of urea. Communications in Soil Science and Plant Analysis, 48(1), 107–112.
Silber, A., Levkovitch, I., & Graber, E. R. (2010). Ph-dependent mineral release and surface properties of cornstraw biochar: Agronomic implications. Environmental Science & Technology, 44(24), 9318–9323.
Zhang, A., Bian, R., Hussain, Q., Li, L., Pan, G., Zheng, J., et al. (2013). Change in net global warming potential of a rice–wheat cropping system with biochar soil amendment in a rice paddy from China. Agriculture, Ecosystems & Environment, 173, 37–45.
Saarnio, S., Heimonen, K., & Kettunen, R. (2013). Biochar addition indirectly affects n2o emissions via soil moisture and plant n uptake. Soil Biology and Biochemistry, 58, 99–106.
Case, S. D. C., Uno, H., Nakajima, Y., Jensen, L. S., & Akiyama, H. (2017). Bamboo biochar does not affect paddy soil n2o emissions or source following slurry or mineral fertilizer amendment-a 15n tracer study. Journal of Plant Nutrition and Soil Science.
Knowles, R. (1982). Denitrification. Microbiological Reviews, 46(1), 43–70.
Dong, Z., Zhu, B., Hua, K., & Jiang, Y. (2015). Soil science and plant nutrition linkage of n 2 o emissions to the abundance of soil ammonia oxidizers and denitrifiers in purple soil under long-term fertilization. Soil Science and Plant Nutrition, ahead-of-print(5), 1-9.
Yang, L., Zhang, X., & Ju, X. (2017). Linkage between N2O emission and functional gene abundance in an intensively managed calcareous fluvo-aquic soil. Science Report, 7, 43283.
Tao, R., Wakelin, S. A., Liang, Y., Hu, B., & Chu, G. (2018). Nitrous oxide emission and denitrifier communities in drip-irrigated calcareous soil as affected by chemical and organic fertilizers. Science of the Total Environment, 612, 739–749.
Espenberg, M., Truu, M., Mander, Ü., Kasak, K., Nõlvak, H., Ligi, T., et al. (2018). Differences in microbial community structure and nitrogen cycling in natural and drained tropical peatland soils. Scientific Reports, 8(1), 4742.
Dandie, C. E., Burton, D. L., Zebarth, B. J., Henderson, S. L., Trevors, J. T., & Goyer, C. (2008). Changes in bacterial denitrifier community abundance over time in an agricultural field and their relationship with denitrification activity. Applied and Environmental Microbiology, 74(19), 5997–6005.
Wang, Y., Uchida, Y., Shimomura, Y., Akiyama, H., & Hayatsu, M. (2017). Responses of denitrifying bacterial communities to short-term waterlogging of soils. Scientific Reports, 7(1), 803.
Chen, Y., Zhou, W., Li, Y., Zhang, J., Zeng, G., Huang, A., & Huang, J. (2014). Nitrite reductase genes as functional markers to investigate diversity of denitrifying bacteria during agricultural waste composting. Applied Microbiology and Biotechnology, 98(9), 4233–4243.
Penton, C. R., Derek, S. L., Amanda, P., Cole, J. R., Liyou, W., Yiqi, L., et al. (2015). Denitrifying and diazotrophic community responses to artificial warming in permafrost and tallgrass prairie soils. Frontiers in Microbiology, 6.
Yang, Y. D., Hu, Y. G., Wang, Z. M., & Zeng, Z. H. (2018). Variations of the nirS-, nirK-, and nosZ-denitrifying bacterial communities in a northern Chinese soil as affected by different long-term irrigation regimes. Environmental Science & Pollution Research, 25(14), 14057–14067.
Lee, S. H., & Kang, H. (2015). The activity and community structure of total bacteria and denitrifying bacteria across soil depths and biological gradients in estuary ecosystem. Applied Microbiology and Biotechnology, 100(4), 1999–2010.
Li, F., Li, M., Shi, W., Li, H., Sun, Z., & Gao, Z. (2017). Distinct distribution patterns of proteobacterial nirk- and nirs-type denitrifiers in the yellow river estuary, China. Canadian Journal of Microbiology, cjm-2017-0053.
Huanhuan, W., Xu, L., Xiang, L., Xinyu, L., Jian, W., Huiwen, Z., et al. (2017). Changes of microbial population and n-cycling function genes with depth in three Chinese paddy soils. Plos one, 12(12), e0189506.
Lehmann, J., & Joseph, S. (2009). Biochar for environmental management: An introduction. In J. Lehmann & S. Joseph (Eds.), Biochar for environmental management: Science and technology (pp. 1–12). London: Earthscan.
Luo, X. Q., Chen, Z., Hu, R. G., Wu, M. N., & Wei, W. X. (2010). Effect of long-term fertilization on the diversity of nitrite reductase genes (nirK and nirS) in paddy soil. Environmental Science, 31(2), 423–430 (In Chinese).
Ishii, S., Ohno, H., Tsuboi, M., Otsuka, S., & Senoo, K. (2011). Identification and isolation of active n2o reducers in rice paddy soil. The ISME Journal, 5(12), 1936–1945.
Yamane, & Tsuyoshi. (2013). Denitrifying bacterial community in manure compost pellets applied to an Andosol upland field. Soil Science and Plant Nutrition, 59(4), 572–579.
Medhi, K., Singhal, A., Chauhan, D. K., & Thakur, I. S. (2017). Investigating the nitrification and denitrification kinetics under aerobic and anaerobic conditions by, paracoccus denitrificans, ISTOD1. Bioresource Technology, 242, 334–343 S0960852417303565.
Carlson, C. A., & Ingraham, J. L. (1983). Comparison of denitrification by pseudomonas stutzeri, pseudomonas aeruginosa, and paracoccus denitrificans. Applied and Environmental Microbiology, 45(4), 1247–1253.
Becker, A., Bergès, H., Krol, E., Bruand, C., & Batut, J. (2004). Global changes in gene expression in sinorhizobium meliloti 1021 under microoxic and symbiotic conditions. Molecular Plant-Microbe Interactions, 17(3), 292–303.
Torres, M. J., Rubia, M. I., Bedmar, E. J., & Delgado, M. J. (2011). Denitrification in sinorhizobium meliloti. Biochemical Society Transactions, 39(6), 1886–1889.
Wang, Y. Y., Lu, S. E., Xiang, Q. J., Yu, X. M., Zhao, K., Zhang, X. P., Tu, S. H., & Gu, Y. F. (2017). Responses of N2O reductase gene(nosZ) denitrifier communities to long term fertilization follow a depth pattern in calcareous purplish paddy soil. Journal of Integrative Agriculture, 16(11), 2597–2611.
Philippot, L., Spor, A., Hénault, C., Bru, D., Bizouard, F., Jones, C. M., Sarr, A., & Maron, P. A. (2013). Loss in microbial diversity affects nitrogen cycling in soil. The ISME Journal, 7(8), 1609–1619.
Braker, G., Dörsch, P., & Bakken, L. R. (2012). Genetic characterization of denitrifier communities with contrasting intrinsic functional traits. FEMS Microbiology Ecology, 79(2), 542–554.
He, J. Z., Zheng, Y., Chen, C. R., He, Y. Q., & Zhang, L. M. (2008). Microbial composition and diversity of an upland red soil under long-term fertilization treatments as revealed by culture-dependent and culture-independent approaches. Journal of Soil Sediments., 8, 349–358.
Hu, Y., Xia, Y., Sun, Q., Liu, K., Chen, X., Ge, T., Zhu, B., Zhu, Z., Zhang, Z., & Su, Y. (2018). Effects of long-term fertilization on, phoD-harboring bacterial community in Karst soils. Science of the Total Environment, 628-629, 53–63.
Funding
The present study was financially supported by the National Key Research and Development Plan of China (Grant No. 2017YFD0800101).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 24.1 kb)
Rights and permissions
About this article
Cite this article
Wang, Y., Qi, L., Huang, R. et al. Characterization of Denitrifying Community for Application in Reducing Nitrogen: a Comparison of nirK and nirS Gene Diversity and Abundance. Appl Biochem Biotechnol 192, 22–41 (2020). https://doi.org/10.1007/s12010-020-03250-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-020-03250-9