Abstract
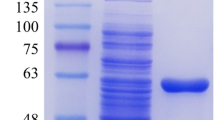
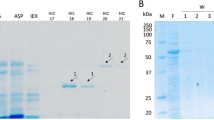
Feruloyl esterase (FAE) is a critical enzyme in bio-extraction of ferulic acid (FA) from plant cell wall. A new FAE (EpFAE1) encoding gene was isolated from Eupenicillium parvum and heterologously expressed in Pichia pastoris cells. Based on phylogenetic tree analysis, the protein EpFAE1 belongs to type A of the seventh FAE subfamily. Using methyl ferulate as substrate, the optimum temperature and pH for the catalytic activity of EpFAE1 were 50 °C and 5.5, respectively. The enzyme exhibited high stability at 50 °C, in a wide pH range (3.0–11.0), or in the presence of 2 M of NaCl. Together with the endo-xylanase EpXYN1, EpFAE1 released 72.32% and 4.00% of the alkali-extractable FA from de-starched wheat bran (DSWB) or de-starched corn bran (DSCB), respectively. Meanwhile, the substrates were pretreated with 1.75% (for DSWB) or 1.0% (for DSCB) of phosphoric acid (PA) at 90 °C for 12 h, followed by enzymatic hydrolysis of the soluble and insoluble fractions. The release efficiencies of FA were up to 84.64% for DSWB and 66.73% for DSCB. Combined dilute PA pretreatment with enzymatic hydrolysis is a low-cost and highly efficient method for the extraction of FA from cereal brans.








Similar content being viewed by others
Abbreviations
- FAE:
-
feruloyl esterase (ferulic acid esterase)
- MFA:
-
methyl ferulate
- FA:
-
ferulic acid
- SDS-PAGE:
-
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- DSWB:
-
de-starched wheat bran
- DSCB:
-
de-starched corn bran
- PA:
-
phosphoric acid
- HPLC:
-
high-performance liquid chromatography
- MW:
-
molecular weight
References
Oliveira, D. M., Mota, T. R., Oliva, B., Segato, F., Marchiosi, R., Ferrarese-Filho, O., Faulds, C. B., & Dos Santos, W. D. (2019). Feruloyl esterases: Biocatalysts to overcome biomass recalcitrance and for the production of bioactive compounds. Bioresource Technology, 278, 408–423.
Dilokpimol, A., Makela, M. R., Aguilar-Pontes, M. V., Benoit-Gelber, I., Hilden, K. S., & de Vries, R. P. (2016). Diversity of fungal feruloyl esterases: updated phylogenetic classification, properties, and industrial applications. Biotechnology for Biofuels, 9, 231.
Crepin, V. F., Faulds, C. B., & Connerton, I. F. (2004). Functional classification of the microbial feruloyl esterases. Applied Microbiology and Biotechnology, 63(6), 647–652.
Gopalan, N., Rodriguez-Duran, L. V., Saucedo-Castaneda, G., & Nampoothiri, K. M. (2015). Review on technological and scientific aspects of feruloyl esterases: A versatile enzyme for biorefining of biomass. Bioresource Technology, 193, 534–544.
Udatha, D. B., Kouskoumvekaki, I., Olsson, L., & Panagiotou, G. (2011). The interplay of descriptor-based computational analysis with pharmacophore modeling builds the basis for a novel classification scheme for feruloyl esterases. Biotechnology Advances, 29(1), 94–110.
Kumar, N., & Pruthi, V. (2014). Potential applications of ferulic acid from natural sources. Biotechnology Reports, 4, 86–93.
Chandrasekharaiah, M., Thulasi, A., Bagath, M., Kumar, D. P., Santosh, S. S., Palanivel, C., Jose, V. L., & Sampath, K. T. (2011). Molecular cloning, expression and characterization of a novel feruloyl esterase enzyme from the symbionts of termite(Coptotermes formosanus) gut. BMB Reports, 44(1), 52–57.
Dilokpimol, A., Makela, M. R., Varriale, S., Zhou, M., Cerullo, G., Gidijala, L., Hinkka, H., Bras, J. L. A., Jutten, P., Piechot, A., Verhaert, R., Hildén, K. S., Faraco, V., & de Vries, R. P. (2018). Fungal feruloyl esterases: Functional validation of genome mining based enzyme discovery including uncharacterized subfamilies. New Biotechnology, 41, 9–14.
Chen, X., Guo, Y., Jia, G., Zhao, H., Liu, G., & Huang, Z. (2019). Ferulic acid regulates muscle fiber type formation through the Sirt1/AMPK signaling pathway. Food&Function, 10(1), 259–265.
Shirai, A., Watanabe, T., & Matsuki, H. (2017). Inactivation of foodborne pathogenic and spoilage micro-organisms using ultraviolet-A light in combination with ferulic acid. Letters in Applied Microbiology, 64(2), 96–102.
Ranum, P., Pena-Rosas, J. P., & Garcia-Casal, M. N. (2014). Global maize production, utilization, and consumption. Annals of the New York Academy of Sciences, 1312, 105–112.
Bento-Silva, A., Vaz Patto, M. C., & do Rosario Bronze M. (2018). Relevance, structure and analysis of ferulic acid in maize cell walls. Food Chemistry, 246, 360–378.
Wang, L., Ma, Z., Du, F., Wang, H., & Ng, T. B. (2014). Feruloyl esterase from the edible mushroom Panus giganteus: a potential dietary supplement. Journal of Agricultural and Food Chemistry, 62(31), 7822–7827.
Topakas, E., Vafiadi, C., & Christakopoulos, P. (2007). Microbial production, characterization and applications of feruloyl esterases. Process Biochemistry, 42(4), 497–509.
Levasseur, A., Navarro, D., Punt, P. J., Belaich, J. P., Asther, M., & Record, E. (2005). Construction of engineered bifunctional enzymes and their overproduction in Aspergillus niger for improved enzymatic tools to degrade agricultural by-products. Applied and Environmental Microbiology, 71(12), 8132–8140.
Wu, H., Li, H., Xue, Y., Luo, G., Gan, L., Liu, J., Mao, L., & Long, M. (2017). High efficiency co-production of ferulic acid and xylooligosaccharides from wheat bran by recombinant xylanase and feruloyl esterase. Biochemical Engineering Journal, 120, 41–48.
Agger, J., Vikso-Nielsen, A., & Meyer, A. S. (2010). Enzymatic xylose release from pretreated corn bran arabinoxylan: differential effects of deacetylation and deferuloylation on insoluble and soluble substrate fractions. Journal of Agricultural and Food Chemistry, 58(10), 6141–6148.
Zhou, X., & Xu, Y. (2019). Integrative process for sugarcane bagasse biorefinery to co-produce xylooligosaccharides and gluconic acid. Bioresource Technology, 282, 81–87.
Amnuaycheewa, P., Hengaroonprasan, R., Rattanaporn, K., Kirdponpattara, S., Cheenkachorn, K., & Sriariyanun, M. (2016). Enhancing enzymatic hydrolysis and biogas production from rice straw by pretreatment with organic acids. Industrial Crops and Products, 87, 247–254.
Kundu, C., & Lee, J. W. (2016). Bioethanol production from detoxified hydrolysate and the characterization of oxalic acid pretreated Eucalyptus (Eucalyptus globulus) biomass. Industrial Crops and Products, 83, 322–328.
Nair, R. B., Kalif, M., Ferreira, J. A., Taherzadeh, M. J., & Lennartsson, P. R. (2017). Mild-temperature dilute acid pretreatment for integration of first and second generation ethanol processes. Bioresource Technology, 245, 145–151.
Nair, R. B., Lundin, M., Brandberg, T., Lennartsson, P. R., & Taherzadeh, M. J. (2015). Dilute phosphoric acid pretreatment of wheat bran for enzymatic hydrolysis and subsequent ethanol production by edible fungi Neurospora intermedia. Industrial Crops and Products, 69, 314–323.
Dilokpimol, A., Makela, M. R., Mansouri, S., Belova, O., Waterstraat, M., Bunzel, M., de Vries, R. P., & Hilden, K. S. (2017). Expanding the feruloyl esterase gene family of Aspergillus niger by characterization of a feruloyl esterase, FaeC. New Biotechnology, 37(Pt B), 200–209.
Li, X., Guo, J., Hu, Y., Yang, Y., Jiang, J., Nan, F., Wu, S., & Xin, Z. (2019). Identification of a novel feruloyl esterase by functional screening of a soil metagenomic library. Applied Biochemistry and Biotechnology, 187(1), 424–437.
Uraji, M., Tamura, H., Mizohata, E., Arima, J., Wan, K., Ogawa, K., Inoue, T., & Hatanaka, T. (2018). Loop of Streptomyces feruloyl esterase plays an important role in the enzyme's catalyzing the release of ferulic acid from biomass. Applied and Environmental Microbiology, 84(3), e02300–e02317.
Long, L., Ding, D., Han, Z., Zhao, H., Lin, Q., & Ding, S. (2016). Thermotolerant hemicellulolytic and cellulolytic enzymes from Eupenicillium parvum 4-14 display high efficiency upon release of ferulic acid from wheat bran. Journal of Applied Microbiology, 121(2), 422–434.
Jiang, K., Li, L., Long, L., & Ding, S. (2018). Comprehensive evaluation of combining hydrothermal pretreatment (autohydrolysis) with enzymatic hydrolysis for efficient release of monosaccharides and ferulic acid from corn bran. Industrial Crops and Products, 113, 348–357.
Long, L., Xu, M., Shi, Y., Lin, Q., Wang, J., & Ding, S. (2018). Characterization of two new endo-beta-1,4-xylanases from Eupenicillium parvum 4-14 and their applications for production of feruloylated oligosaccharides. Applied Biochemistry and Biotechnology, 186(4), 816–833.
Kumar, S., Stecher, G., Li, M., Knyaz, C., & Tamura, K. (2018). MEGA X: Molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution, 35(6), 1547–1549.
Robert, X., & Gouet, P. (2014). Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Research, 42(Web Server issue), W320–W324.
Gong, Y. Y., Yin, X., Zhang, H. M., Wu, M. C., Tang, C. D., Wang, J. Q., & Pang, Q. F. (2013). Cloning, expression of a feruloyl esterase from Aspergillus usamii E001 and its applicability in generating ferulic acid from wheat bran. Journal of Industrial Microbiology & Biotechnology, 40(12), 1433–1441.
de Vries, R. P., Michelsen, B., Poulsen, C. H., Kroon, P. A., van den Heuvel, R. H., Faulds, C. B., Williamson, G., van den Hombergh, J. P., & Visser, J. (1997). The faeA genes from Aspergillus niger and Aspergillus tubingensis encode ferulic acid esterases involved in degradation of complex cell wall polysaccharides. Applied and Environmental Microbiology, 63(12), 4638–4644.
Nieter, A., Kelle, S., Linke, D., & Berger, R. G. (2016). Feruloyl esterases from Schizophyllum commune to treat food industry side-streams. Bioresource Technology, 220, 38–46.
Long, L., Zhao, H., Ding, D., Xu, M., & Ding, S. (2018). Heterologous expression of two Aspergillus niger feruloyl esterases in Trichoderma reesei for the production of ferulic acid from wheat bran. Bioprocess and Biosystems Engineering, 41(5), 593–601.
Ewert, J., Gluck, C., Strasdeit, H., Fischer, L., & Stressler, T. (2018). Influence of the metal ion on the enzyme activity and kinetics of PepA from Lactobacillus delbrueckii. Enzyme and Microbial Technology, 110, 69–78.
Uraji, M., Arima, J., Inoue, Y., Harazono, K., & Hatanaka, T. (2014). Application of two newly identified and characterized feruloyl esterases from Streptomyces sp. in the enzymatic production of ferulic acid from agricultural biomass. PLoS One, 9(8), e104584.
Zeng, Y., Yin, X., Wu, M. C., Yu, T., Feng, F., Zhu, T. D., & Pang, Q. F. (2014). Expression of a novel feruloyl esterase from Aspergillus oryzae in Pichia pastoris with esterification activity. Journal of Molecular Catalysis B: Enzymatic, 110, 140–146.
Hunt, C. J., Tanksale, A., & Haritos, V. S. (2016). Biochemical characterization of a halotolerant feruloyl esterase from Actinomyces spp.: refolding and activity following thermal deactivation. Applied Microbiology and Biotechnology, 100(4), 1777–1787.
Yu, P., Maenz, D. D., McKinnon, J. J., Racz, V. J., & Christensen, D. A. (2002). Release of ferulic acid from oat hulls by Aspergillus ferulic acid esterase and Trichoderma xylanase. Journal of Agricultural and Food Chemistry, 50(6), 1625–1630.
Feher, C., Gal, B., Feher, A., Barta, Z., & Reczey, K. (2015). Investigation of commercial enzyme preparations for selective release of arabinose from corn fibre. Journal of Chemical Technology and Biotechnology, 90(7), 1329–1337.
Agger, J., Johansen, K. S., & Meyer, A. S. (2011). pH catalyzed pretreatment of corn bran for enhanced enzymatic arabinoxylan degradation. New Biotechnology, 28(2), 125–135.
Acknowledgments
The authors thank Dr. Franz St. John from the USDA Forest Service for improving the manuscript.
Funding
This work was supported by grants from a 948 Research Project (No. 2013-4-16) from the State Forestry Administration of China, a research project (30370043) from the National Natural Science Foundation of China, the Jiangsu Provincial Government Scholarship for Overseas Studies from Jiangsu Provincial Department of Education, China, a Science and Technology Project of Guizhou Province in China (Grant No. [2019]2333), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1037 kb)
Rights and permissions
About this article
Cite this article
Long, L., Wu, L., Lin, Q. et al. Highly Efficient Extraction of Ferulic Acid from Cereal Brans by a New Type A Feruloyl Esterase from Eupenicillium parvum in Combination with Dilute Phosphoric Acid Pretreatment. Appl Biochem Biotechnol 190, 1561–1578 (2020). https://doi.org/10.1007/s12010-019-03189-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-019-03189-6